
Presentation, history and investigations
A 70-year-old male with hypertension and type 2 diabetes mellitus (T2DM) was infected with SARS-CoV-2 in late 2020, before COVID-19 vaccines became available in Hong Kong. He developed severe COVID-19, which required immediate noninvasive ventilation.
Investigations performed on presentation showed very high viral load (nasopharyngeal swab [NPS] cycle threshold [Ct] value, 17.55). The patient also had neutropenia (neutrophils, 5.8 x 109/L), low lymphocyte count (0.6 x 109/L) and high lactate dehydrogenase (LDH; 243 U/L), and a severe cytokine storm triggered by the infection was suspected. Chest X-ray revealed bilateral lower lobe infiltration of the lungs, while CT scan showed diffused ground-glass opacity.
Treatment and rehabilitation
Intravenous (IV) remdesivir (200 mg loading dose on day 1, followed by 100 mg QD from day 2) and subcutaneous interferon (IFN) beta-1b (16 million IU all day) were started upon hospital admission (on days 4–5 from symptom onset). Dexamethasone (6 mg Q24H IV) was started together with the antiviral treatment to control the cytokine storm. IV piperacillin-tazobactam (4 g/0.5 g) was given on day 3 of hospitalization for coverage of nosocomial infection.
On day 3 of hospitalization, the patient was admitted to the intensive care unit (ICU) and required full intubation and ventilator support due to severe pneumonia. He also developed pneumothorax that required chest drain during his ICU stay.
Baricitinib (4 mg QD) and prophylactic subcutaneous enoxaparin were started after a week in ICU.
The patient was clear of the virus at the end of week 3, despite having developed SARS-CoV-2 antibodies at around week 2 in hospital. He received remdesivir and IFN beta-1b for 7 days, dexamethasone for 3 weeks, baricitinib for 10 days, tocilizumab (480 mg IV) for two doses, and enoxaparin for >1 month until mobilization. He did not experience any septic shock, deep vein thrombosis or pulmonary embolism.
The patient recovered with extensive lung fibrosis due to ventilator support and pneumothorax, and was given three courses of the oral antifibrotic agent, nintedanib. He also had poor lower limb strength due to ICU-related neuropathy, and required intensive physiotherapy for 6 months (inpatient basis followed by outpatient physiotherapy). He was hospitalized for a total of 5 months (4 weeks in ICU, 4 weeks in acute ward, and 3 months in rehabilitation ward). Upon discharge, he required home oxygen support for >1 year.
Currently, the patient is able to walk unaided for 300–400 m, no longer requires oxygen support, and has resumed working.
Discussion
Patients with higher risks of COVID-19 hospitalization and severe disease include those of older age, and those with underlying medical conditions such as T2DM, hypertension, cardiovascular disease, kidney disease, obesity, chronic lung disease and a compromised immune system, such as our patient.1-3
In high-risk patients, earlier initiation of antiviral treatment within the optimal window (within 3–5 days from symptom onset) ensures timely inhibition of viral replication and can help reduce the risks of cytokine storm and severe disease. Timely addition of immunomodulators (such as dexamethasone) ± a second agent (such as baricitinib) for suppression of hyperinflammation has also been found to be important.4,5
Remdesivir (for 3–5 days) is currently indicated in patients with SARS-CoV-2 infection who do not require oxygen support and are considered to need treatment due to risk factors for COVID-19 progression, or in patients with COVID-19 pneumonia (for 5 days).6 For hospitalized patients with moderate or moderate-to-severe COVID-19 who require supplemental oxygen, and those with COVID-19 pneumonia requiring supplemental oxygen who have impending respiratory failure and high viral load (reverse transcription [RT]-PCR Ct value <25), remdesivir is recommended by the Hospital Authority (HA) as an antiviral treatment of choice unless contraindicated (ALT >5 times upper limit of normal).7
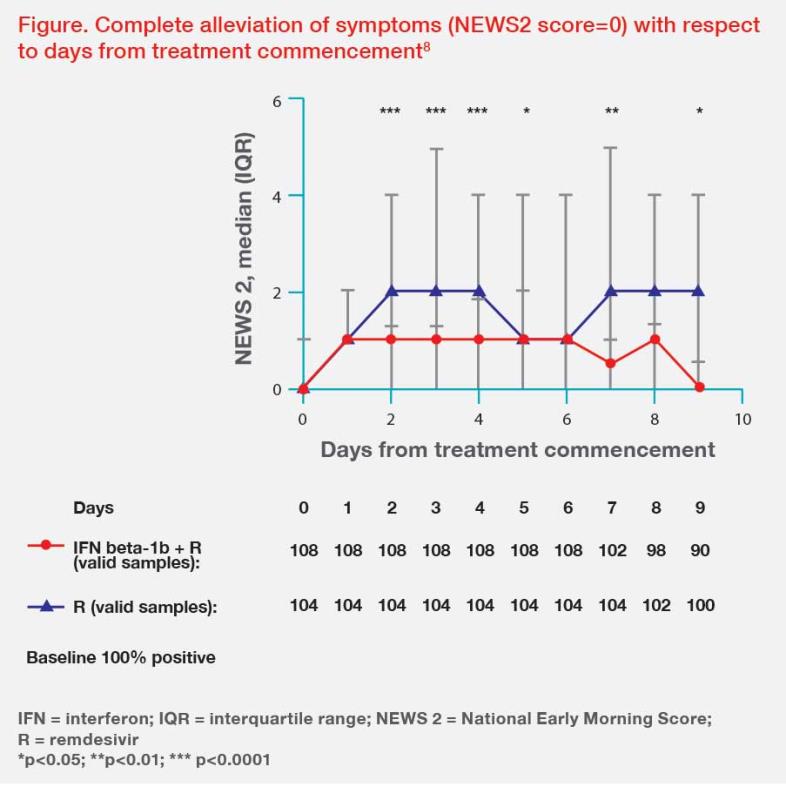
Clinically, remdesivir can be used in combination with IFN beta-1b or ritonavir-boosted nirmatrelvir in patients with severe COVID-19.8-10 Our study in 212 high-risk hospitalized COVID-19 patients (median age, 65 years; comorbid chronic disease, 75 percent) showed that early treatment (at a median of 3 days from symptom onset) with remdesivir and IFN beta-1b led to significantly faster complete alleviation of symptoms (4 days vs 6.5 days; hazard ratio [HR], 6.59; 95 percent confidence interval [CI], 6.10–7.09; p<0.0001), NPS viral negativity (6 days vs 8 days; HR, 8.16; 95 percent CI, 7.79–8.52; p<0.0001), and immunoglobulin G seropositivity (8 days vs 10 days; HR, 10.78; 95 percent CI, 9.98–11.58; p<0.0001) vs remdesivir alone.8 (Figure) Patients treated with remdesivir plus IFN beta-1b also had significantly shorter hospitalization (5 days vs 7 days; HR, 7.38; 95 percent CI, 6.88–7.87; p<0.0001) and ICU stay (8 days vs 11 days; HR, 11.06; 95 percent CI, 8.54–13.63; p<0.001) vs remdesivir alone.8 Our initial experience also suggests a better treatment effect with remdesivir in combination with ritonavir-boosted nirmatrelvir vs remdesivir alone in hospitalized patients with severe COVID-19.9
Remdesivir’s IV route of administration is also an advantage for patients who cannot tolerate or are unable to take oral antiviral drugs (eg, some elderly patients, patients who require mechanical ventilation), and patients who missed the optimal window for oral antiviral treatment.7,11
Our patient with severe COVID-19 benefitted from early treatment with remdesivir, IFN beta-1b and dexamethasone, as recommended by HA.7 Hong Kong’s cohorting and isolation measures through 2020–2021 enabled early diagnosis, hospitalization and treatment of COVID-19, resulting in better patient outcomes than in the US or UK during the same period, where only very sick patients were hospitalized. With the availability of oral antivirals against COVID-19, both the US National Institute of Health (NIH) and UK National Institute for Health and Care Excellence (NICE) have recently incorporated recommendations for early antiviral treatment in COVID-19 patients.12,13
The importance of vaccination cannot be overemphasized, especially in persons at high risk of severe COVID-19. High-risk individuals infected with SARS-CoV-2 should be started on antiviral treatment as early as possible to supress viral load and reduce the risk of cytokine storm, which helps reduce the risk of subsequent complications. In patients with poor lung function after viral clearance, rehabilitative physiotherapy should continue until lung function recovery, and antifibrotic therapy should be prescribed to those with lung fibrosis.