Achieving low-risk status in PAH with prostacyclin receptor agonist–based triple therapy




Presentation, investigation and treatment
A 72-year-old female with a history of well-controlled hypertension presented with unexplained dyspnoea and reduced exercise tolerance for the past 2–3 years.
Echocardiography in June 2018 showed raised systolic pulmonary arterial pressure (PAP) of 94 mm Hg and increased right ventricular (RV) size. Chest radiography revealed cardiomegaly and pulmonary trunk dilatation, which led to suspicion of pulmonary arterial hypertension (PAH). Right heart catheterization (RHC) in June 2018 revealed combined pre- and post-capillary pulmonary hypertension with mean PAP of 50 mm Hg, pulmonary vascular resistance (PVR) of 7 WU, and pulmonary arterial wedge pressure of 27 mm Hg.
With the left-heart disease being managed, the patient received furosemide 40 mg QD for PAH, which yielded only a modest response. Therefore, double combination therapy with a phosphodiesterase type 5 inhibitor (PDE5i; sildenafil 20 mg TID) and an endothelin receptor antagonist (ERA; macitentan 10 mg QD) was initiated in August 2018, which led to marked symptom improvement.
In November 2022, the patient experienced disease progression with further increased dyspnoea, reduced exercise tolerance, and marked limitation of physical activity (WHO functional class [FC], III). As the patient required oxygen supplementation, she became homebound, and her 6-minute walking distance (6MWD) was 190 m. Findings were consistent with intermediate–high risk status.1
The oral selective prostacyclin receptor agonist selexipag 200 mg BID was added to sildenafil and macitentan on 7 December 2022, and gradually uptitrated to 600 mg BID (maximum tolerated dose).2 The patient responded well to selexipag, with 6MWD improving to 267 m after 1 month of treatment. Her N-terminal pro-B type natriuretic peptide (NT-proBNP) level was 157 ng/L after 2 months. Notably, the patient reported significant symptom improvement after 4 months of treatment, which enabled her to discontinue oxygen supplementation at home. Her WHO FC also improved from III to II.
During the selexipag uptitration period, the patient was admitted to hospital for adverse event (AE) monitoring. She experienced grade 1 headache and diarrhoea at the beginning of treatment, which were managed with analgesics and antidiarrhoeal agents.
Last seen on 4 January 2024, the patient remained clinically stable with selexipag-based triple therapy. Her latest risk assessment further improved to a low-risk status.
Discussion
Definitive PAH diagnosis can be challenging within Hong Kong’s public healthcare system due to the extended waiting time for a Doppler echocardiography, which is needed to guide further invasive RHC. Given that the initial symptoms of PAH are nonspecific (eg, dyspnoea), it is important to remain vigilant and consider incorporating findings from electrocardiography, NT-proBNP levels, cardiopulmonary exercise testing, chest X-ray, and CT thorax to help narrow down differential diagnoses before a Doppler echocardiogram can be obtained.1
The 2022 European Society of Cardiology (ESC)/European Respiratory Society (ERS) Guidelines for the diagnosis and treatment of pulmonary hypertension advocate a risk-based, goaloriented treatment approach, with the key objective of achieving and maintaining a low-risk status in patients through regular follow-up assessments.1 In this patient, the treatment decision to add selexipag upon disease progression was based on class IIA recommendation of the ESC/ERS guideline and GRIPHON to achieve a low-risk status.1,3
GRIPHON was a phase III, randomized trial on PAH that investigated the efficacy and safety of selexipag vs placebo in treatment-naive patients and patients who were receiving ERA and/or PDE5i at baseline (n=1,156).3,4 Like our patient, the majority of patients (52.5 percent) in the trial had WHO FC III symptoms at baseline.3
Adding selexipag to double combination therapy significantly reduced the risk of the primary endpoint of the first adjudicated event of all-cause death, complications from worsening of PAH, or disease progression up to 7 days after the last intake of selexipag or placebo (hazard ratio [HR], 0.63; 95 percent confidence interval [CI], 0.44–0.90) vs placebo.4 The significant treatment effect was partially driven by a reduction in hospitalization for worsening of PAH, which was consistent with our patient’s experience of remaining admission-free for >14 months after selexipag initiation.3
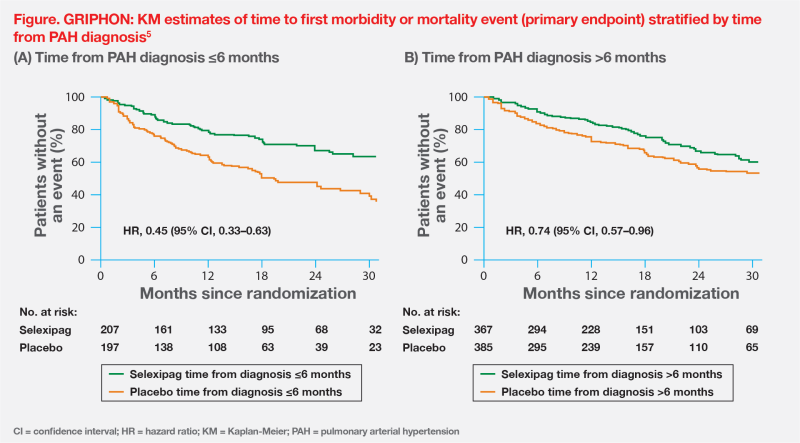
GRIPHON also reinforced the importance of early initiation of PAH-specific therapy from the time of diagnosis. A post hoc analysis showed that selexipag’s benefit on primary morbidity or mortality events was more pronounced in those who initiated treatment ≤6 months vs after >6 months of PAH diagnosis (risk reduction, 55 percent vs 26 percent; pinteraction=0.0219).5 (Figure)
In our centre’s experience, patients with better WHO FC (eg, II–III) tend to benefit notably from triple therapy. However, the benefits may be less pronounced in those with WHO FC IV, underscoring the importance of early treatment intensification upon disease progression.
Recent real-world evidence showed WHO FC improvement with selexipag treatment. The SPHERE registry collected data on routine clinical use of selexipag in the US.6 Same as the GRIPHON population, most patients had WHO FC III symptoms at baseline (51.0 percent).3,6 After a median follow-up of 17.8 months, WHO FC improved in 20.2 percent, 22.0 percent and 24.9 percent of patients at 6,12 and 18 months, respectively, regardless of whether selexipag was newly initiated (≤60 days before enrolment) or previously initiated (>60 days).6 Our patient’s FC improvement from III to II is consistent with these data.
Furthermore, our patient's 6MWD improved by 77 m after 1 month of add-on selexipag treatment, which was consistent with the significant improvement demonstrated in GRIPHON.3 However, longer-term 6MWD data for our patient were not available at the time of writing.
While addition of selexipag to a treatment regimen within 6 months of PAH diagnosis may confer greater clinical benefits, patients in GRIPHON and SPHERE received selexipag at an average of >2 years after diagnosis.5,6 There is much room for improvement in early treatment escalation if a positive change in risk status is not achieved during regular assessment. In conclusion, our case and the GRIPHON trial illustrate the importance of early diagnosis, regular risk stratification, and early targeting of the prostacyclin pathway with an oral agent in optimizing patient outcomes.