Adjuvant PD-1 ICI in an elderly patient with MIUC ineligible for chemotherapy
Specialist in Clinical Oncology
Private practice
Hong Kong

Presentation, treatment and response
A 79-year-old retired female physician, who was a nonsmoker, presented with painless gross haematuria in September 2022. She had type 2 diabetes mellitus (T2DM), hyper-cholesterolaemia and fatty liver disease, which were well controlled with maintenance oral antihyperglycaemic agents and statin therapy.
In October 2022, cytoscopy and biopsy showed urothelial carcinoma in situ (CIS) on the left side of the urinary bladder extending to the posterior wall and the roof. CT showed patchy mural thickening with increased contrast enhancement of the mucosal layer along the left side wall of the urinary bladder extending to the posterior wall and roof, as well as patchy increase in contrast enhancement of the urothelial lining of the left mid-ureter. The patient was given intravesical Bacillus Calmette-Guérin (BCG) from November to December 2022.
In January 2023, cystoscopy showed a 4 cm tumour at the left posterior wall extending to the left lateral wall of the bladder and left ureteroscopy showed half-circumferential growth over the left mid-ureter. Bladder and left ureteric biopsies showed high-grade papillary urothelial carcinoma (UC). CT scans confirmed absence of distant metastases.
In February 2023, the patient underwent transurethral resection of bladder tumour. Pathologic findings showed muscle-invasive UC (MIUC).
On 20 March 2023, she underwent left laparoscopic nephroureterectomy, radical cystectomy, hysterectomy, bilateral salpingo-oophorectomy, and ileal conduit urinary diversion. Final pathologic findings showed CIS in distal ureter and high-grade muscle-invasive bladder cancer (MIBC) with perivesical tissue invasion, consistent with pT3N0M0 or stage IIIA disease. PD-L1 combined positive score was <1.
The patient consulted our clinic for further management. At this time, her Eastern Cooperative Oncology Group performance status (ECOG PS) was 2. She was considered unfit for cisplatin-based chemotherapy due to her PS status and a calculated creatinine clearance rate of 47 mL/min, indicating impaired renal function.1,2
On 27 April 2023, the patient started adjuvant therapy with the PD-1 immune checkpoint inhibitor (ICI), nivolumab (240 mg Q2W intravenously).1 After two initial doses, she complained of malaise, constipation, and vaginal discharge, which may have been postoperative complications. Treatment was temporarily withheld and her symptoms gradually improved. She resumed nivolumab treatment on 8 June 2023. For her convenience, nivolumab regimen was changed to 480 mg Q4W in July 2023.
In August 2023, CT scan showed no evidence of recurrence. The patient’s ECOG PS improved to 1. She experienced fluctuating glucose level in October 2023, which was controlled by addition of insulin to her T2DM medications. She continued to tolerate nivolumab well, with satisfactory quality of life. Adjuvant nivolumab is to be continued for a total of 1 year.
Discussion
The current standard of care for MIBC includes neoadjuvant cisplatin-based chemotherapy for eligible patients, followed by radical cystectomy. Our patient was unfit for cisplatin-based chemotherapy due to advanced age, renal insufficiency and ECOG PS of 2.1,2
After cystectomy, patients are offered adjuvant therapy based on pathologic risk. For patients at high risk of recurrence (ie, pT3–pT4 or pN+ disease not treated with neoadjuvant cisplatin), options include adjuvant cisplatin-based chemotherapy or nivolumab monotherapy.1 However, high-risk MIUC patients who received neoadjuvant cisplatin are only eligible for nivolumab monotherapy in the adjuvant setting.1
Approval of adjuvant nivolumab in MIUC is based on results of CheckMate 274.1,3-6 This randomized, double-blind, placebo-controlled trial previously met its dual primary endpoint of disease-free survival (DFS) with nivolumab vs placebo, both in the intention-to-treat (ITT) population and inpatients with PD-L1 tumour proportion score (TPS) ≥1 percent. DFS benefit was seen in most subgroups analyzed, including age, sex, ECOG PS, nodal status, prior cisplatin-based chemotherapy, and PD-L1 status.3,5,7
After an extended minimum follow-up of 31.6 months, nivolumab continued to show DFS benefits both in the ITT (22.0 months vs 10.9 months; hazard ratio [HR], 0.71; 95 percent confidence interval [CI], 0.58–0.86) and MIBC (25.6 months vs 8.5 months; HR, 0.63; 95 percent CI, 0.51 0.78) populations.4,5 (Figure) DFS benefit was seen in most analyzed subgroups, including prior cisplatin-based chemotherapy (yes: HR, 0.54; 95 percent CI, 0.40–0.72; no: HR, 0.91; 95 percent CI, 0.70–1.17) and PD-L1 expression (≥1: HR, 0.53; 95 percent CI, 0.38–0.73; <1: HR, 0.84; 95 percent CI, 0.66–1.06).5 The DFS benefit was also observed both in patients with PD-L1 ≥1 percent (HR, 0.44; 95 percent CI, 0.30–0.63) and PD-L1 <1 percent (HR, 0.74; 95 percent CI, 0.56–0.97) within the MIBC population.4
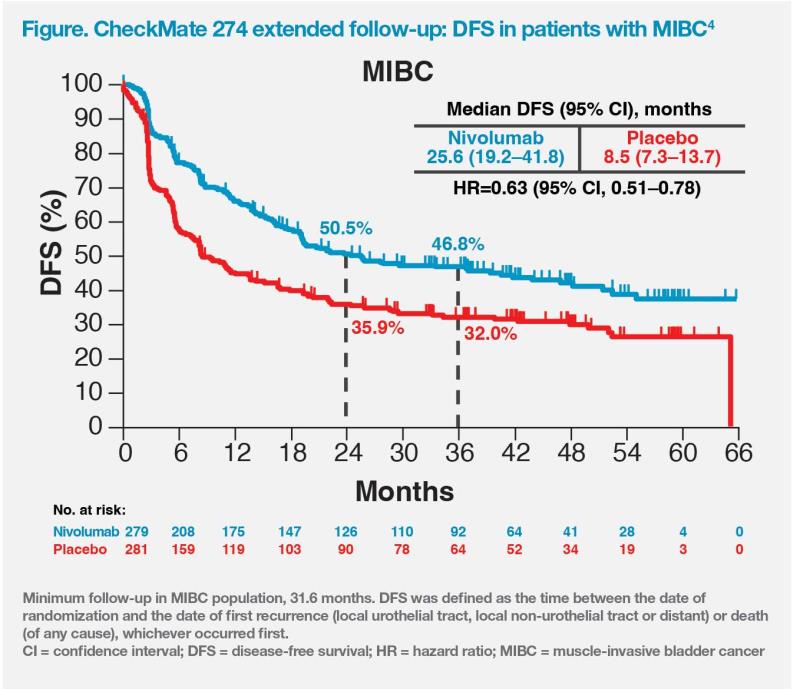
The CheckMate 274 findings support the use of adjuvant nivolumab after radical cystectomy in patients with high-risk MIBC (eg, pT3 but no nodal involvement or metastasis, such as our patient) regardless of PD-L1 status.3-5 Since UC primarily occurs in ex-smokers, current smokers or the elderly who are more likely to have impaired renal function and poor PS, many patients may be ineligible for cisplatin, but may still benefit from adjuvant nivolumab, which offers an effective and tolerable chemotherapy-free alternative.1-5
Nivolumab’s safety profile in CheckMate 274 was consistent with that reported in previous trials involving patients with metastatic urothelial carcinoma and other cancers.3-5,8-10 Pruritus, fatigue, diarrhoea, and rash (any grade: 23.1 percent, 17.4 percent, 16.8 percent, and 15.1 percent, respectively) were the most frequently reported nivolumab-related adverse events (AEs).3
Interstitial lung disease or pneumonitis is a less common nivolumab-related AE that requires vigilance for relevant symptoms.11 The fluctuating glucose level experienced by our diabetic patient may have been due to nivolumab, which is associated with immune-mediated endocrinopathies in some cases, underscoring the importance of a multidisciplinary approach in management of patients with comorbidities.11
Before nivolumab, effective and well-tolerated adjuvant treatment options for elderly patients with high-risk UC were lacking, since a majority of these patients are unfit for chemotherapy due to poor PS and comorbidities. Extended follow-up results of CheckMate 274 demonstrate nivolumab’s survival benefits and acceptable tolerability even in elderly patients with MIBC, regardless of PD-L1 status and previous neoadjuvant cisplatin-based chemotherapy.3-5,11