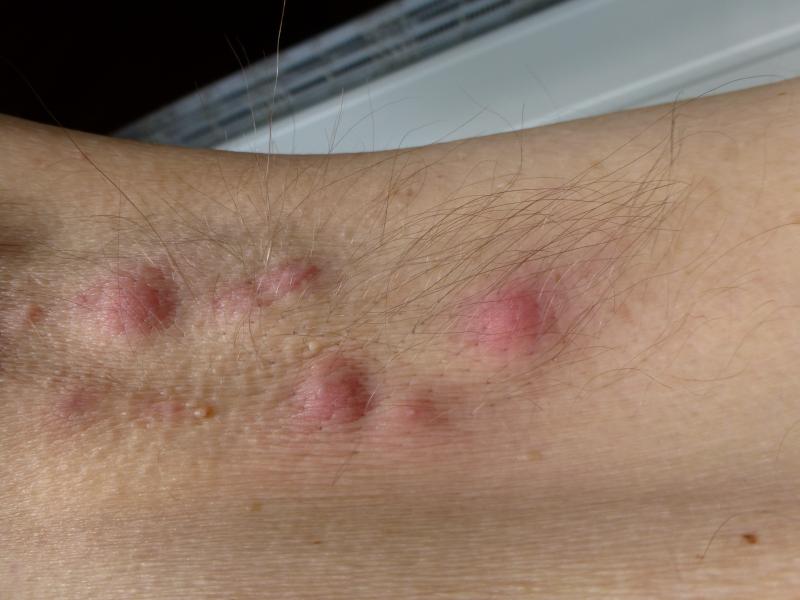
Interleukin (IL)-1α inhibition with bermekimab appears to be safe and effective in the treatment of moderate-to-severe hidradenitis suppurativa (HS), inducing clinical response without raising substantial safety concerns, according to the results of a phase II trial.
The trial included 42 HS patients, among whom 24 had previously failed anti-TNF therapy and 18 were anti-TNF naïve. All patients were subcutaneously administered bermekimab at 400 mg weekly (13 doses).
There were no treatment-related adverse events (AEs) recorded except for injection site reactions. A total of 58 nonserious AEs occurred, with most being grade 1 (59 percent) or 2 (36 percent). Two serious AEs were documented (grade 3 fall and grade 3 HS pain), none of which were related to bermekimab, and none led to treatment discontinuation.
In terms of efficacy, 61 percent of patients in the anti-TNF naïve group and 63 percent of those in the anti-TNF failure group achieved HS clinical response after 12 weeks of treatment. There were marked reductions in abscesses and inflammatory nodules seen in 60 percent (p<0.004) and 46 percent (p<0.001) of patients in the respective groups.
Furthermore, those experiencing pain showed clinically and statistically significant pain relief. The Visual Analogue Scale pain score decreased by 64 percent (p<0.001) in the anti-TNF naïve group and by 54 percent (p<0.001) in the anti-TNF failure group.
The present data point to IL-1α inhibition as an important clinical target for skin disease, with bermekimab potentially representing a new therapeutic option for managing moderate-to-severe HS, researchers said.