Brolucizumab in DME provides durable visual and anatomical improvements with favourable safety profile
Swiss Eye Institute in Bern and Rotkreuz
Switzerland
Anti–vascular endothelial growth factor (anti-VEGF) agents are currently recommended for patients with diabetic macular oedema (DME), but treatment regimens are associated with high medical burden that may compromise compliance and visual outcomes. At a Novartis-sponsored meeting, Professor Justus Garweg of the Swiss Eye Institute in Bern and Rotkreuz, Switzerland, shared personal experiences on advantages of the anti-VEGF, brolucizumab, in patients with DME while highlighting 2-year results of the pivotal phase III KESTREL and KITE trials.
Personal experience with brolucizumab
“In our clinical practice, patients with DME experienced favourable functional and anatomic outcomes with brolucizumab similar to those observed in the pivotal, 100-week, phase III, double-blind KESTREL and KITE trials, including patients with multiorgan disease having mild diabetic retinopathy progression and uncomplicated DME, and those with advanced nonproliferative diabetic retinopathy,” shared Garweg. [Am J Ophthalmol 2023;doi:10.1016/j.ajo.2023.07.012]
“Notably, anatomic improvements are achieved early with brolucizumab, with macular oedema frequently resolving by the end of the loading phase,” he added. (Figure) “This is important since persistent macular oedema has been associated with lower visual gains and a higher treatment demand in patients with DME.” [Eye (Lond) 2020;34:480-490]
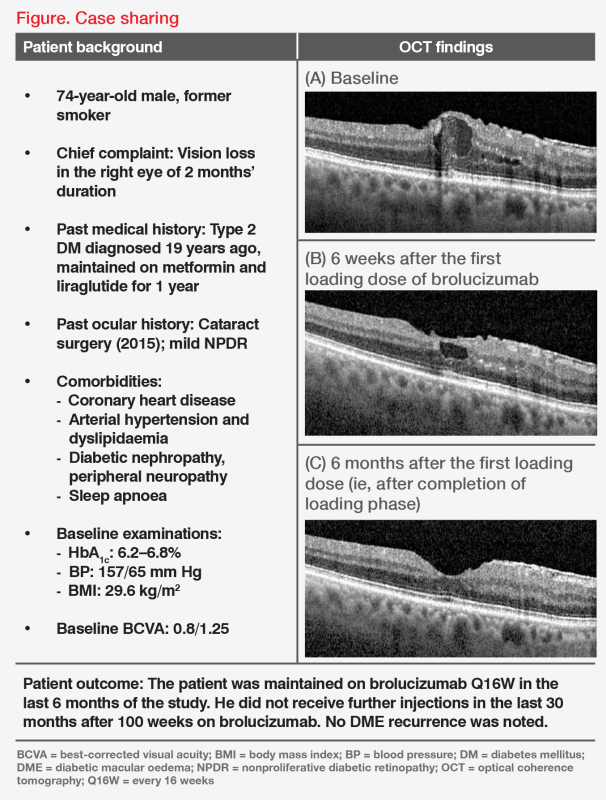
“These improvements are also usually sustained, consistent with the durability observed in KESTREL and KITE,” he continued. “Many patients with DME are also able to take the Q16W dosing regimen, or may even stop treatment without recurrence over 2–3 years after 2 years of continued treatment with no new safety signals.” [Am J Ophthalmol 2023;doi:10.1016/j.ajo.2023.07.012]
KESTREL and KITE trials
“In the KESTREL and KITE trials, we evaluated the efficacy and safety of intravitreal anti-VEGF injections of brolucizumab vs aflibercept in patients aged ≥18 years with type 1 or 2 DM [HbA1c ≤10 percent] with visual impairment due to DME [ie, best-corrected visual acuity (BCVA) letter score range, 23– 78],” said Garweg. [Am J Ophthalmol 2022;238:157-172; Am J Ophthalmol 2023;doi:10.1016/j.ajo.2023.07.012]
Study design
Participants received either brolucizumab 3 mg or 6 mg or aflibercept 2 mg in KESTREL (n=566), or brolucizumab 6 mg or aflibercept 2 mg in KITE (n=360). In both trials, the brolucizumab regimen consisted of five loading doses at Q6W intervals followed by Q12W dosing, with optional adjustment to Q8W if disease activity was identified at predefined assessment visits. In KITE, at week 72, treatment intervals could be extended to Q16W in the brolucizumab arm, based on disease stability assessments. The aflibercept regimen in both trials consisted of five loading doses at Q4W intervals followed by fixed Q8W dosing.
Results
After 52 weeks, primary endpoint results showed noninferior visual gains with brolucizumab 6 mg vs aflibercept 2 mg. These results were sustained at week 100, with brolucizumab 6 mg achieving +8.8 change in BCVA letter vs +10.6 for aflibercept 2 mg in KESTREL, and +10.9 vs +8.4 in KITE.
In both studies, brolucizumab treatment resulted in greater reductions in central subfield thickness (CSFT) from baseline (ie, least squares mean differences in CSFT, brolucizumab 6 mg vs aflibercept: KESTREL, -3.5 μm; KITE, -23.2 μm). In addition, fewer patients on brolucizumab had intraretinal fluid (IRF)/subretinal fluid (SRF) vs those on aflibercept. At week 100 in KESTREL, the proportions of eyes with IRF/SRF were 45.8 percent and 41.8 percent in the brolucizumab 3 mg and 6 mg arms, respectively, vs 54.0 percent in the aflibercept arm (brolucizumab 6 mg vs aflibercept difference: -12.4 percent; 95 percent confidence interval [CI], -22.8 to -2.1). In KITE, the respective proportions were 40.8 percent vs 56.9 percent (difference: -16.2 percent; 95 percent CI, -26.4 to -5.9). [Am J Ophthalmol 2023;doi:10.1016/j.ajo.2023.07.012]
These results were achieved by 32.9 percent (KESTREL) and 47.5 percent (KITE) of patients on brolucizumab 6 mg who were maintained on Q12W and Q12W/Q16W dosing, respectively, suggesting that brolucizumab 6 mg may be more effective in providing deeper and sustained DME control even with extension of dosing intervals up to Q16W vs aflibercept Q8W.
Intraocular inflammation (IOI), including retinal vasculitis and retinal vascular occlusion, are known adverse events associated with brolucizumab. IOI rates were 4.2 percent vs 1.1 percent for brolucizumab 6 mg vs aflibercept in KESTREL, and 2.2 percent vs 1.7 percent in KITE. In KESTREL, retinal vasculitis was reported in 0.5 percent vs none of the patients on brolucizumab 6 mg vs aflibercept. No retinal vasculitis was reported in KITE for either treatment. Retinal vascular occlusion rates were 1.6 percent with brolucizumab 6 mg vs 0.5 percent with aflibercept in KESTREL and 0.6 percent in both treatment arms in KITE. [Beovu Hong Kong Prescribing Information; Am J Ophthalmol 2023;doi:10.1016/j. ajo.2023.07.012]
“In both studies, patients treated with brolucizumab received fewer injections vs aflibercept,” he added. “The median number of total injections through week 100 in KESTREL was 11.0 for both brolucizumab arms vs 15.0 for aflibercept, and 10.0 vs 15.0, respectively, in KITE.”
Conclusions
“Based on KESTREL and KITE results as well as our clinical experience, brolucizumab 6 mg, dosed up to Q16W for up to 2 years, is associated with clinically meaningful vision gains and greater anatomic improvements vs aflibercept, with an overall favourable benefit/risk profile,” said Garweg. “Brolucizumab could therefore provide an additional therapeutic option in DME that may reduce the treatment burden on patients, physicians and the healthcare system.”