Latest CAPTIVATE trial results, which were presented at the 65th American Society of Hematology Annual Meeting and Exposition (ASH 2023) held in San Diego, California, US, show that the all-oral regimen of ibrutinib (a Bruton’s tyrosine kinase [BTK] inhibitor) plus venetoclax (a BCL-2 inhibitor) continues to provide high rates of progression-free survival (PFS) and overall survival (OS) at 5 years of follow-up when used as first-line treatment for chronic lymphocytic leukaemia (CLL)/small lymphocytic lymphoma (SLL), including in patients with high-risk disease features.
Ibrutinib is a once-daily oral BTK inhibitor that has demonstrated PFS and OS benefits in patients with previously untreated CLL/SLL in four randomized phase III studies. These studies compared ibrutinib as monotherapy or in combination with monoclonal antibodies vs standard chemotherapy or chemoimmunotherapy regimens. [Leukemia 2020;34:787-798; N Engl J Med 2019;381:432-443; Lancet Oncol 2019;20:43-56; N Engl J Med 2018;379:2517-2528]
The combination of ibrutinib with venetoclax, an oral inhibitor of the anti-apoptotic protein BCL-2, preferentially targets distinct cell compartments and CLL subpopulations to eliminate both dividing and resting CLL cells through the two agents’ distinct and complementary modes of action. As ibrutinib mobilizes CLL cells out of lymph nodes and other lymphoid niches and into peripheral blood, they become more susceptible to venetoclax-induced apoptosis. At the same time, inhibition of BTK by ibrutinib enhances the dependence of CLL cells on BCL-2, thereby increasing their sensitivity to venetoclax and accelerating apoptosis. [Blood 2022;139:3278-3289]
CAPTIVATE: 1L ibrutinib + venetoclax in CLL/SLL
CAPTIVATE is an international, multicentre, randomized phase II study of ibrutinib plus venetoclax in patients aged ≤70 years with previously untreated CLL/SLL, who were divided into two separate cohorts: a minimal residual disease (MRD)–guided randomized treatment discontinuation cohort and a fixed-duration (FD) treatment cohort. The present report focuses on the FD cohort, which was conducted as an open-label, single-arm trial. [Blood 2022;139:3278-3289]
Patients (n=159) in the FD cohort (age, 18–70 years; Eastern Cooperative Oncology Group performance status, 0–2) received all-oral treatment with three cycles of single-agent ibrutinib (420 mg QD) followed by twelve 28-day cycles of combined ibrutinib (420 mg QD) plus venetoclax (target dose, 400 mg QD after standard 5-week ramp-up, with tumour lysis syndrome [TLS] prophylaxis and monitoring). The FD cohort included patients with high-risk features of unmutated IGHV (uIGHV; 56 percent) or del(17p) and/or TP53 mutation (17 percent).
Primary analysis
At the time of primary analysis, the median time on study was 27.9 months, and 92 percent of patients completed all planned treatment. The primary endpoint of complete response (CR) rate was 56 percent in patients without del(17p) and 55 percent in the all-treated population. Best undetectable MRD rates were 77 percent in peripheral blood (PB) and 60 percent in bone marrow (BM). At 24 months, the PFS rate was 95 percent and the OS rate was 98 percent. [Blood 2022;139:3278-3289]
At baseline, 21 percent of patients were in the high tumour burden category for TLS risk, which reduced to 1 percent following three cycles of ibrutinib lead-in.
The most common treatment-emergent AEs were diarrhoea (62 percent), nausea (43 percent), neutropenia (42 percent), and arthralgia (33 percent). AEs were primarily of grade 1 or 2 in severity. The most common grade 3/4 AEs were neutropenia (33 percent), hypertension (6 percent), and decreased neutrophil count (5 percent). One fatal AE (sudden death) occurred during ibrutinib lead-in. Serious AEs occurred in 23 percent of patients.
“First-line ibrutinib plus venetoclax represents the first all-oral, once daily, chemotherapy-free FD regimen for patients with CLL. FD ibrutinib plus venetoclax achieved deep and durable responses as well as promising PFS, including in patients with high-risk features,” concluded the researchers.
4-year data
At 4 years of follow-up, the best CR rate was 58 percent and overall response rate (ORR) was 96 percent; 4-year PFS and OS rates were 79 percent and 98 percent, respectively. While 4-year PFS rates were numerically lower in patients with uIGHV (73 percent) or del(17p) and/or TP53 mutation (63 percent) vs the overall population (79 percent), the OS rates remained consistently high across all subgroups and in the overall population (97 percent, 96 percent and 98 percent, respectively). [Hemasphere 2023;7:e2822842]
Median time to next treatment (TTNT) was not reached overall, with 84 percent of patients remaining free from next treatment at 4 years. “FD ibrutinib plus venetoclax continues to provide deep, durable remissions with clinically meaningful PFS and time off treatment, including in patients with high-risk disease features,” remarked the researchers.
One case of prostate cancer was reported at 4-year follow-up, as second malignancies continued to be monitored off treatment. “New safety findings off-treatment were expectedly negligible, highlighting the benefits of a FD regimen,” commented the researchers.
5-year update
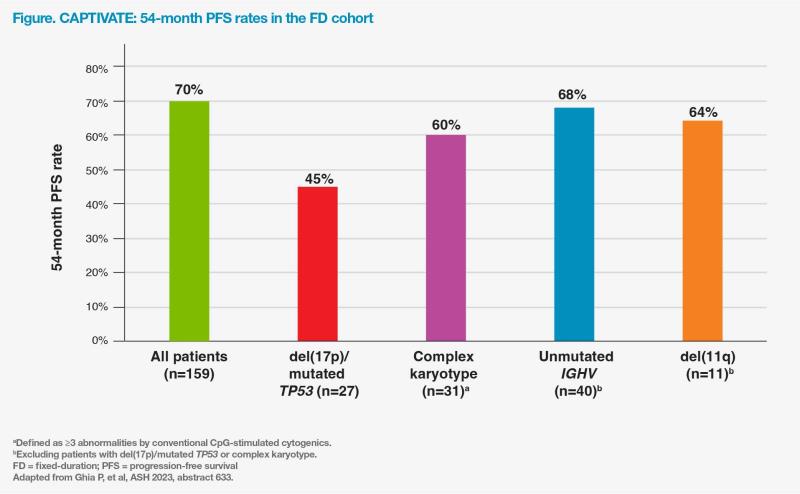
At a median time on study of 56 months, the 54-month PFS and OS rates in the FD cohort were 70 percent and 97 percent, respectively. (Figure) [Ghia P, et al, ASH 2023, abstract 633]
There was no change in best response rates since the 4-year follow-up analysis: the CR rate (including CR with incomplete bone marrow recovery [CRi]) was still 58 percent and ORR was still 96 percent. Median duration of CR/ CRi was not reached in patients who achieved CR/CRi (n=92), of whom 98 percent achieved durable CR/CRi lasting ≥12 cycles.
At 54 months, the PFS rates were promising among most patients with high-risk features (60–68 percent), but this rate was numerically lower in the subset of patients with del(17p)/mutated TP53 (45 percent). (Figure)
In the combined CAPTIVATE population comprising 159 patients in the FD cohort and 43 patients the MRD cohort's placebo arm (15 cycles of FD ibrutinib plus venetoclax), 53 had progressive disease (PD) at 5-year follow-up, with PD occurring >2 years after completion of treatment in the majority of patients (62 percent). Acquired resistance-associated mutation in BCL2 (A113G) occurred in one out of 40 patients with available samples at PD. Clinically relevant mutations in BTK, BCL2, or PLCG2 were not noted in any other patients.
As data on serious AEs considered related to study treatment and second malignancies continued to be collected after completion of FD treatment for an additional year of follow-up, one AE of basal cell carcinoma was reported. Overall, second malignancies were reported in 8 percent of patients since completion of ibrutinib plus venetoclax treatment.
ConclusionDeep remissions with clinically meaningful PFS are still observed with FD ibrutinib plus venetoclax, including in patients with high-risk genomic features, at up to 5 years of follow-up.