Cardiorenal protection with an SGLT2 inhibitor in a patient with T2DM and multiple comorbidities
Specialist in Endocrinology, Diabetes & Metabolism
Caritas Medical Centre
Hong Kong
History and presentation
A 65-year-old female overweight security guard with a 10-year history of type 2 diabetes mellitus (T2DM) was referred to our centre from a general outpatient clinic in 2019 due to suboptimal glycaemic control (HbA1c, 8–9 percent) since diagnosis.
The patient had multiple cardiorenal-metabolic (CRM) comorbidities, including hypertension treated with losartan 100 mg QD and metoprolol 100 mg BID, hypercholesterolaemia treated with rosuvastatin 20 mg QD, ischaemic heart disease treated with percutaneous coronary intervention (PCI) in 2016 and aspirin 80 mg QD, chronic kidney disease (CKD) with macroalbuminuria, and nonproliferative diabetic retinopathy. She also had a strong family history of T2DM – both of her parents and two of three siblings had T2DM, and her father with T2DM died of acute myocardial infarction (MI).
Treatment and response
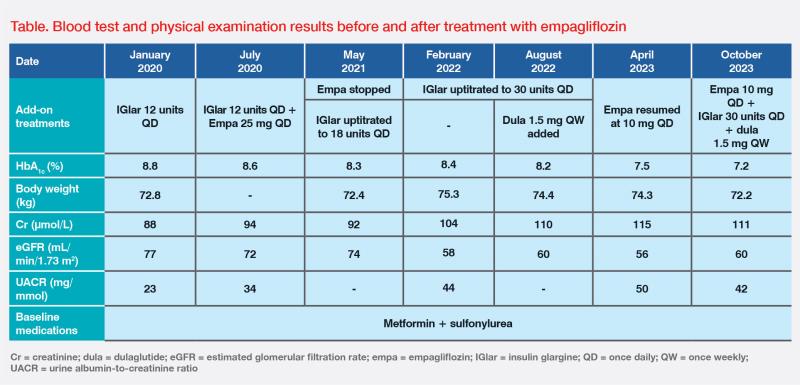
In January 2020, insulin glargine (IGlar) 12 units QD was added to her baseline antidiabetic medications of metformin and sulfonylurea, but her glycaemic control remained suboptimal. (Table) As the patient worked as a security guard, she declined treatment with multiple daily insulin injections due to inconvenience.
The patient was briefly treated with empagliflozin 25 mg QD for 10 months, after which we discontinued it in May 2021 due to only a modest improvement in glycaemic control and the adverse event (AE) of genital mycotic infection. Between May 2021 and August 2022, IGlar was gradually uptitrated to 30 units QD, and a glucagon-like peptide-1 receptor agonist (dulaglutide 1.5 mg QW) was added, which led to an improvement in HbA1c level to 7.5 percent. (Table)
However, her renal function progressively deteriorated, with estimated glomerular filtration rate (eGFR) dropping to 56 mL/min/1.73 m2 and urine albumin-to-creatinine ratio (UACR) increasing to 50 mg/mmol, indicating CKD stage A3G3a. In April 2023, empagliflozin was resumed at a lower dose of 10 mg QD due to its proven cardiorenal-protective effect.1 (Table)
The patient tolerated empagliflozin well this time and did not report any AEs. Last seen in October 2023, her renal function stabilized, with body weight and HbA1c level further improving to 72.2 kg and 7.2 percent, respectively. (Table) The patient did not have further cardiovascular (CV) or renal events, and will remain on empagliflozin for cardiorenal protection.
Discussion
The CV, renal and metabolic systems are interrelated; acute or chronic dysfunction of one of these systems may exert negative effects on the others through haemodynamic, neurohormonal and immunological feedback pathways.1 Nearly half of T2DM patients develop CKD, while one-third have CV disease and more than half die of it.2-4 For example, our T2DM patient developed both ischaemic heart disease and CKD on top of other CRM comorbidities, and her father died of MI.
Sodium-glucose cotransporter 2 (SGLT2) inhibitors (eg, empagliflozin) have transformed the paradigm of treatment of various CRM conditions due to their profound cardiorenal benefits.5 The cardiorenal benefits of empagliflozin were consistently demonstrated in the landmark EMPA-REG OUTCOME (T2DM patients with established CV disease), EMPEROR-Reduced and EMPEROR-Preserved (HF patients across the entire spectrum of ejection fraction), and EMPA-KIDNEY (CKD patients) trials.6-10
In EMPA-REG OUTCOME (n=7,020), empagliflozin significantly lowered the risk of 3-point major adverse CV events (MACE; death from CV causes, nonfatal MI, or nonfatal stroke) vs placebo (hazard ratio [HR], 0.86; 95.02 percent confidence interval [CI], 0.74–0.99; p=0.04). Notably, CV benefits emerged early, with reductions in hospitalization for heart failure (HHF) and CV death observed within weeks, and reductions in all-cause death and 3-point MACE noticed within months of treatment initiation.6 Empagliflozin also significantly reduced incident or worsening nephropathy (HR, 0.61; 95 percent CI, 0.53–0.70; p<0.001) vs placebo in EMPA-REG OUTCOME.7 Additionally, empagliflozin demonstrated a robust reduction in HHF in both EMPEROR-Reduced (n=3,730) and EMPEROR-Preserved (n=5,988), and reduced the risk of CKD progression in EMPA-KIDNEY (n=6,609).8,10,11
The EMPRISE Europe and Asia study in 83,946 pairs of T2DM patients provides real-world evidence on empagliflozin’s cardiorenal benefits. Compared with dipeptidyl peptidase-4 (DPP-4) inhibitors, empagliflozin was associated with lower risks of HHF (HR, 0.70; 95 percent CI, 0.60–0.83), all-cause mortality (HR, 0.55; 95 percent CI, 0.48–0.63), stroke (HR, 0.82; 95 percent CI, 0.71–0.96), and end-stage renal disease (HR, 0.43; 95 percent CI, 0.30– 0.63).12
As shown in this case, some physicians may discontinue SGLT2 inhibitors in T2DM patients due to modest HbA1c reduction. However, SGLT2 inhibitors are not merely glucose-lowering agents, and their cardiorenal protective effects are independent of glycaemic control.13 Potential mechanisms of action (MoA) underlying their cardiorenal benefits may include haemodynamic alterations, intraglomerular pressure reduction, and improved endothelial function.13 As such, empagliflozin should not be discontinued only because of modest HbA1c reduction.
In this patient, optimal early treatment with empagliflozin should have been provided in 2016 (after stabilization of ischaemic heart disease) or in 2019 (after diagnosis of CKD) for cardiorenal protection. The American Diabetes Association (ADA) 2023 guidelines recommended first-line treatment with SGLT2 inhibitors with proven cardiorenal benefits for T2DM patients with established atherosclerotic CV disease or high CV risk, HF, and/or CKD.14
Genital infections are common AEs of SGLT2 inhibitors, but they are generally mild to moderate in severity and manageable.15 Topical clotrimazole cream and hygiene advice were provided to our patient after reinitiation of empagliflozin, after which she tolerated the treatment very well.
SGLT2 inhibitors may cause an initial eGFR dip due to their haemodynamic MoA, but most patients’ eGFR will stabilize afterwards, and treatment should be continued unless the initial eGFR dip is >30 percent or eGFR drops to <20 mL/min/1.73 m2.16
Although rare, diabetic ketoacidosis is a possible complication of SGLT2 inhibitor treatment, especially in patients with acute illness or insulin insufficiency. Patients should follow sick day rules, and SGLT2 inhibitors should be temporarily stopped when fasting, undergoing surgery, or during acute illness.15