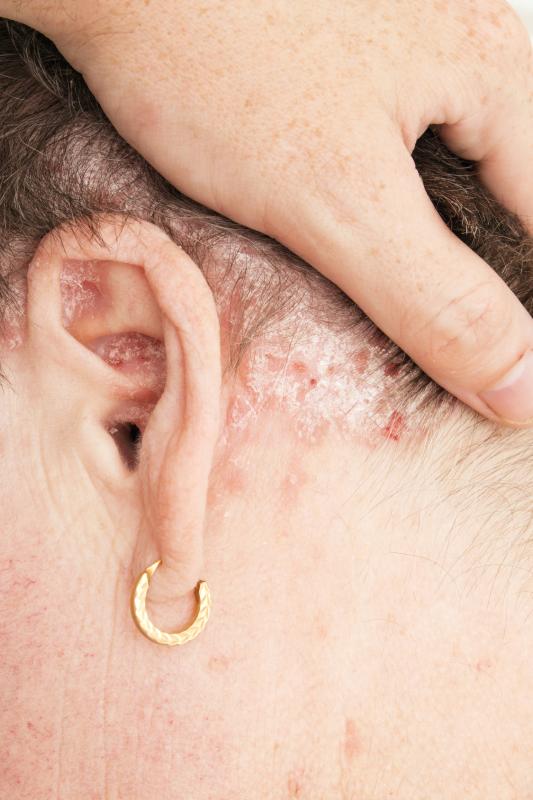
Patients with intertriginous, anogenital or facial psoriasis see significant clinical improvements with the use of crisaborole 2% ointment, a nonsteroidal phosphodiesterase 4 inhibitor, according to the results of a study. The topical agent is also well tolerated.
To assess the safety and efficacy of crisaborole 2% ointment, a double-blind, randomized, vehicle-controlled trial was performed in 21 participants with intertriginous, anogenital and facial psoriasis. They were randomly assigned 2:1 to receive 4 weeks of twice-daily treatment with either crisaborole 2% ointment (n=14) or vehicle ointment (n=7), which was followed by 4 weeks of open-label treatment with crisaborole 2% ointment. Disease severity was measured using the Target Lesion Severity Scale (TLSS).
After 4 weeks, participants in the crisaborole group showed substantially greater improvement compared with those in the vehicle group (66 percent vs 9 percent; p=0.0011). This improvement persisted through the open-label phase, exhibiting 81-percent lesional improvement by week 8. Moreover, 71 percent of participants in the crisaborole group achieved clinical clearance.
No adverse events were reported.
This finding was consistent with that of a previous study, which suggested that the application of crisaborole ointment to sensitive skin areas was well tolerated in healthy volunteers, making it a potential topical treatment alternative for patients with psoriasis or atopic dermatitis. [Am J Clin Dermatol 2016;17:519-526]
The present study was limited to a single tertiary care centre and by its small sample size, according to the investigators