Current treatment considerations for precision medicine: Bridging scientific data to clinical practice
Center for Integrated Oncology
University of Cologne, Germany

Precision medicine and tumour-agnostic approach are changing the landscape of non-small-cell lung cancer (NSCLC) and many other advanced solid tumours without satisfactory standard treatments. In an industry-sponsored meeting endorsed by the Hong Kong Society of Clinical Oncology, Professor Jürgen Wolf of the Center for Integrated Oncology, University of Cologne, Germany, lead investigator of an international phase II trial that led to the approval of capmatinib for advanced NSCLC harbouring MET exon 14 (METex14) skipping mutation, discussed the importance of panel molecular testing and targeted therapy in different solid tumours, focusing in particular on advanced NSCLC with actionable driver mutations, such as MET alterations.
Precision medicine and tumour-agnostic management approach
While older anticancer therapies have traditionally focused on specific tumour types, the newer approach of precision medicine seeks to target individual cancer genomic profiles irrespective of the tumour site. These “tumour-agnostic” targeted therapies may be used to treat any cancer with a particular molecular alteration or a biomarker. In line with this approach, evidence for new drug approvals increasingly comes from smaller patient groups with varying tumour types with a common genomic change.
Combination of the BRAF kinase inhibitor dabrafenib and MEK inhibitor trametinib is an example of a tumour-agnostic treatment approved on the basis of ROAR (Rare Oncology Agnostic Research), NCI-MATCH (National Cancer Institute-Molecular Analysis for Therapy Choice) and Study X2102 basket trial results. [Acta Med Acad 2022;51:217-231]
Targeting BRAFV600E mutation in advanced cancers
BRAF missense kinase domain mutations, the vast majority of which are V600E, occur in approximately 8 percent of human tumours. These mutations are observed most frequently in melanoma, but are also found in tumours of the lung, colon, thyroid and other sites. [Curr Top Microbiol Immunol 2012:355:83-98]
ROAR was a phase II basket trial (n=206) of dabrafenib plus trametinib in patients with BRAFV600E-mutated advanced rare cancers: anaplastic thyroid carcinoma, biliary tract cancer, gastrointestinal stromal tumour, adenocarcinoma of the small intestine, low-grade glioma, high-grade glioma, hairy cell leukaemia, and multiple myeloma. The overall response rates (ORRs) were >50 percent in most cohorts. (Figure 1) [Nat Med 2023;29:1103-1112]
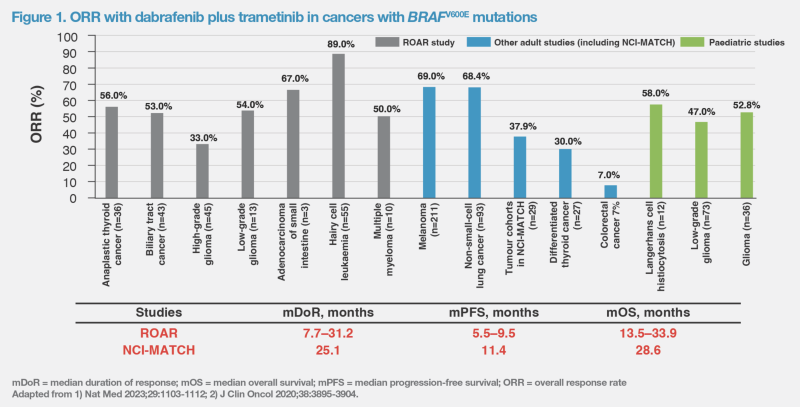
The NCI-MATCH trial was designed to explore whether targeted therapies for specific genetic changes would be effective regardless of the tumour type. Subprotocol H of the trial was an open-label, single-arm study (n=29) that evaluated dabrafenib and trametinib in patients whose tumours had a BRAFV600E mutation (excluding melanoma, thyroid or colorectal cancers, where use of dabrafenib and trametinib has been evaluated extensively in earlier published studies). In evaluable patients, ORR was 37.9 percent and median duration of response (mDoR) was 25.1 months. (Figure 1) [J Clin Oncol 2020;38:3895-3904]
The combination of dabrafenib and trametinib received accelerated US FDA approval in June 2022 for treatment of adult and paediatric patients ≥6 years of age with unresectable or metastatic solid tumours with BRAFV600E mutation that progressed following prior treatment and have no satisfactory alternative options. [Nat Med 2023;29:1103-1112; https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-dabrafenib-combination-trametinib-unresectable-or-metastatic-solid] The combination is not indicated for colorectal cancer, due to its known intrinsic resistance to BRAF inhibition. (Figure 1)
Other tumour-agnostic agents approved by US FDA so far are pembrolizumab for tumours with high microsatellite instability or mutational burden, larotrectinib and entrectinib for cancers with NTRK fusions, and selpercatinib for RET-fusion tumours.
“The steady increase in the number of tumour-agnostic targeted treatment options underscores the importance of the broad use of next-generation sequencing [NGS] in cancer diagnostics to fully take advantage of the benefits of precision medicine,” highlighted Wolf.
Molecular profiling in NSCLC
Following the discovery of several targetable driver mutations, treatment of lung adenocarcinoma is becoming increasingly molecularly guided. “Molecular profiling is key to molecularly guided therapy. Given the increasing number of targetable aberrations, there is no diagnostic alternative to NGS,” said Wolf.
In Hong Kong, free comprehensive genomic profiling testing for stage IV NSCLC was introduced to the public sector in 2022. Initial data from the programme revealed less common mutations amenable to treatment with targeted therapies in a sizeable proportion of patients.
Capmatinib in advanced NSCLC with METex14
One of the rare targetable mutations involves activation of the MET pathway, which may be caused by different mechanisms (ie, overexpression, gene amplification and METex14 skipping mutations). [N Engl J Med 2020;383:944-957] Currently, the only MET tyrosine kinase inhibitors (TKIs) approved for use in Hong Kong are capmatinib and tepotinib. [https:// www.drugoffice.gov.hk/eps/do/en/ consumer/reg_pharm_products/index. html]
Capmatinib is an oral, ATP-competitive, highly selective and reversible inhibitor of MET kinase with >10,000-fold selectivity for MET receptor kinase vs other human kinases, and is the most potent inhibitor of METex14 kinase. [Clin Cancer Res 2019;25:3164-3175; Clin Cancer Res 2011;17:7127-7138; J Thorac Oncol 2019;14:1753-1765]
The efficacy of capmatinib (400 mg BID) in patients with MET-dysregulated advanced NSCLC was demonstrated in GEOMETRY mono-1, a multiple-cohort, phase II study. In treatment-naïve patients (cohorts 5b and 7; n=60), the ORR was 68.3 percent, disease control rate (DCR) was 98.3 percent, mDoR was 16.6 months, median progression-free survival (mPFS) was 12.5 months, and median overall survival (mOS) was 25.5 months. In patients with METex14 skipping mutation, capmatinib was more effective in the first-line than in pretreated/relapsed setting, although even in later-line setting, capmatinib demonstrated ORR of 44 percent and 36 percent of patients had DoR ≥12 months. [Wolf J, et al, ASCO 2021, abstract 9020; Wolf J, et al, ELCC 2022, abstract 26P]
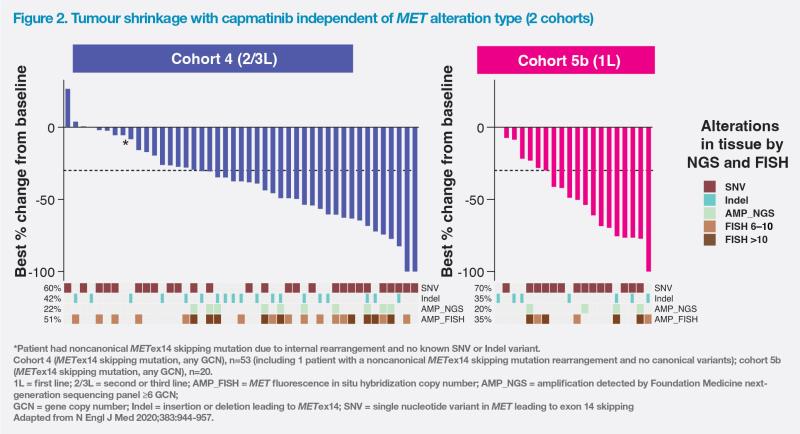
Based on GEOMETRY mono-1 data, observed antitumour activity and mDoR seemed to be independent of MET alteration type leading to METex14 skipping mutation and independent of the co-occurrence of MET amplification (METamp). (Figure 2) [N Engl J Med 2020;383:944-957]
The most frequently reported adverse events in the study across all cohorts were peripheral oedema (51 percent) and nausea (45 percent), but these were mostly grade 1 or 2 and reversible. [N Engl J Med 2020;383:944-957]
Real-world experience with capmatinib in 81 patients with advanced METex14 NSCLC reflects GEOMETRY mono-1 data. ORR was 68 percent in treatment-naïve patients and 50 percent in pretreated patients, while mPFS was 10.6 months and 9.1 months, respectively. After a median follow-up of 11.0 months, mOS was not reached in treatment-naïve patients and 17.2 months in pretreated patients. [Ther Adv Med Oncol 2022; doi:10.1177/17588359221103206]
“Capmatinib also demonstrated a remarkable effect on brain metastases,” added Wolf. “Of 13 patients with METex14 skipping mutation and brain involvement in GEOMETRY mono-1, 12 had intracranial disease control based on neuroradiologic assessment. Seven patients had an intracranial response, including four who had complete resolution of all brain lesions.” [N Engl J Med 2020;383:944- 957]
Results of a retrospective medical record review confirm capmatinib’s efficacy in NSCLC patients with METex14 skipping mutation and brain metastases. Of 68 eligible patients, 80.9 percent received capmatinib, 16.2 percent received immunotherapy (IO)–containing regimens and 2.9 percent received chemotherapy alone as their first-line therapy after brain metastasis diagnosis. In the overall cohort, the intracranial real-world ORR was 87.3 percent with capmatinib and 27.3 percent with IO-containing regimens. Respective real-world PFS was 14.1 months and 5.1 months, with corresponding 1-year PFS rates of 70.8 percent and 18.2 percent. [Future Oncol 2023;19:217-228]
IO +/- chemo or MET TKI in METex14 NSCLC: Which is better?
There is currently no study that directly compares chemotherapy +/- IO with a MET TKI for patients with stage IV NSCLC and METex14 skipping mutation. KEYNOTE-189, which compared pemetrexed and carboplatin/cisplatin (platinum doublet) plus placebo vs pembrolizumab (anti–PD-1 monoclonal antibody) plus platinum doublet in an unselected population of patients without EGFR/ALK alterations, yielded respective ORRs of 19.9 percent and 48.3 percent and respective mOS of 10.6 months and 22.0 months. [J Clin Oncol 2023;41:1992-1998]
Phase II results from relatively small trials of first-line capmatinib or tepotinib in NSCLC patients with MET mutations reported ORRs of 55–70 percent and mOS of approximately 24 months. [N Engl J Med 2020;383:944-957; N Engl J Med 2020;383:931-943]
Although some METex14 tumours have moderate-to-high PD-(L)1 expression, retrospective studies suggest that PD-(L)1 inhibitor activity is diminished in METex14 NSCLC. Survival outcomes did not improve among those with higher PD-(L)1 tumour scores and tumour mutational burden. (Figure 3)
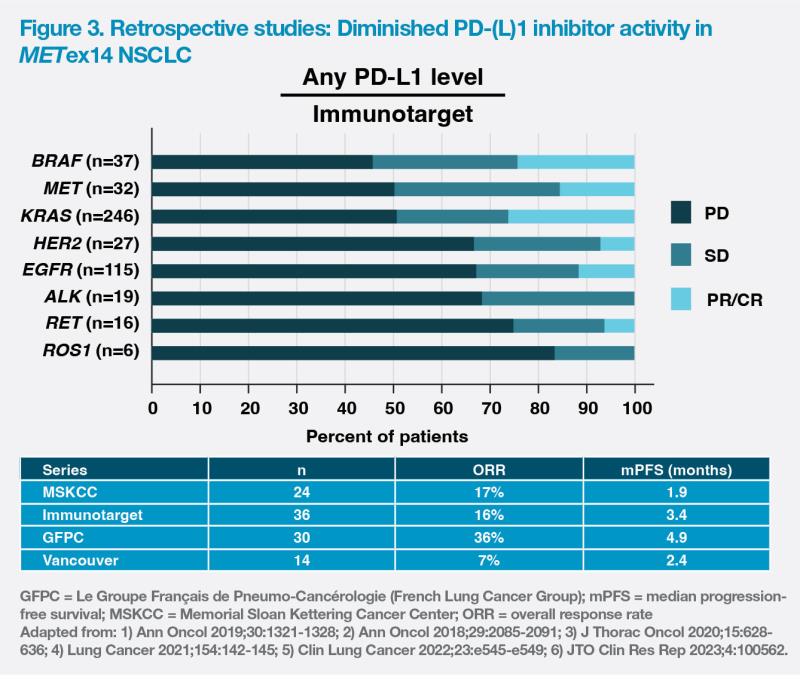
Real-world evidence appears to confirm that patients with advanced METex14 mutant NSCLC may not benefit from IO monotherapy. Analysis of data from 337 patients with MET-altered NSCLC revealed that while mOS of patients with METamp tumours was significantly longer with immune checkpoint inhibitor therapy vs chemotherapy (19.0 months vs 8.0 months; p<0.0001), there was no significant difference in patients with METex14. [J Thorac Oncol 2021;16:572-582]
“As it is unclear whether data from IO trials can be applied to METex14 patients or whether IO is effective at all in METex14 NSCLC, my preferred option is using a MET TKI in the first line,” said Wolf.

Conclusion
“Acceptance of precision medicine and the growing armamentarium of tumour-agnostic treatment require testing for mutations, including very rare ones. Repeated biopsies will help to clarify the molecular background of resistance and aid in molecularly guided therapy [in multiple cancer types],” said Wolf. “In case of lung cancer, it is clear from many clinical trials that broad NGS testing should be done for all patients as early as possible as well as in relapse.”