Immunotherapy (IO) has become part of standard care for early-stage non-small-cell lung cancer (NSCLC), necessitating a multidisciplinary approach for optimum treatment. At a symposium conducted during the Hong Kong Thoracic Society and CHEST Autumn Respiratory Seminar (HKTS/CHEST ARS) 2023, Mr Naveed Alam, Consultant Thoracic Surgeon at St. Vincent Hospital in Melbourne, Australia, shared promising results of the IMpower010 trial of adjuvant atezolizumab, emphasized the importance of early involvement of multidisciplinary teams (MDTs) in patient care, and discussed surgeons’ concerns regarding neoadjuvant IO, including high surgical attrition rate, fibrosis, and adhesions.
Why do surgeons care about IO?
While surgery is an important modality in treatment of early-stage lung cancer, the prognosis of stage IIA–IIIA patients remains suboptimal, with 5-year overall survival (OS) rates of 36–60 percent and high recurrence rates of 58–63 percent. [J Thorac Oncol 2016;11:39-51; J Natl Cancer Inst 2015;107:djv059]
“Surgery alone is clearly not enough. More treatments are needed to further improve patients’ outcomes. This is where adjuvant IO comes into play,” said Alam.
In the phase III IMpower010 trial, 1,005 patients with completely resected stage IB (tumours ≥4 cm)–IIIA NSCLC per the Union Internationale Contre le Cancer and American Joint Committee on Cancer staging system (7th edition) were randomized 1:1 to receive adjuvant intravenous (IV) atezolizumab 1,200 mg Q3W for 16 cycles or best supportive care (BSC) after ≤4 cycles of cisplatin-based chemotherapy. In stage II–IIIA patients with high PD-L1 expression (tumour cell [TC] expression, ≥50 percent), adjuvant atezolizumab significantly reduced the risk of recurrence or death by 57 percent vs BSC (median disease-free survival [DFS], not estimable vs 35.7 months; hazard ratio [HR], 0.43; 95 percent confidence interval [CI], 0.27–0.68) after a median follow-up of 32.2 months. [Lancet 2021;398:1344-1357]
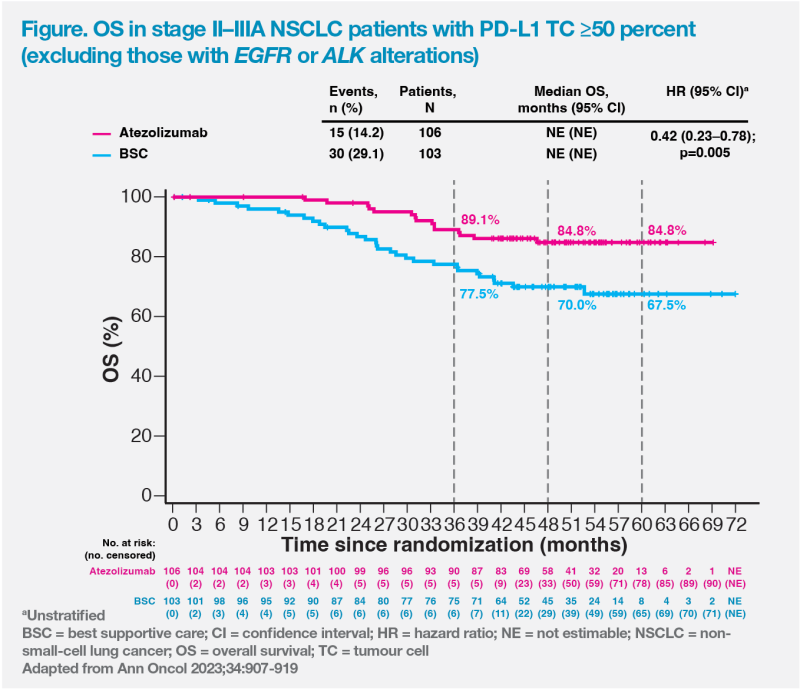
Although OS data were immature, there was a positive trend favouring adjuvant atezolizumab vs BSC, with an impressive 5-year OS rate of 84.8 percent vs 67.5 percent (HR, 0.42; 95 percent CI, 0.23–0.78; p=0.005) in the high PD-L1 expression population without EGFR or ALK alterations. (Figure) [Brunelli A, et al, ESTS 2023, abstract O-026]
Why surgeons’ perspectives matter?
Resectable ≠ operable
To optimize patients’ outcomes, evaluation of resectability and operability as well as the decision on whether to opt for neoadjuvant therapy or upfront surgery ± adjuvant therapy should be based on early MDT discussion with thoracic surgeons, oncologists, pulmonologists, and other specialists. [Cancer Treat Res Commun 2022:31:100538]
“Neoadjuvant therapy [and surgery] should be considered only in patients with both resectable and operable tumours,” pointed out Alam. “Attention to detail on resectability and operability is necessary in preoperative care of NSCLC. These two terms are not synonyms and should not be confused.” [Thorac Surg Clin 2021;31:379-391]
Resectability refers to feasibility of achieving complete resection, which is mostly affected by tumour-related factors, such as tumour size and stage, as well as involvement of nearby structures (eg, aorta). Operability refers to patients’ suitability for and tolerability of surgery, which are mostly affected by patient-related factors, including predicted postoperative forced expiratory volume in 1 second (FEV1), predicted postoperative carbon monoxide diffusing capacity (DLCO), age, presence of distant metastases, and overall health and fitness. [Alam N, HKTS/CHEST ARS 2023]
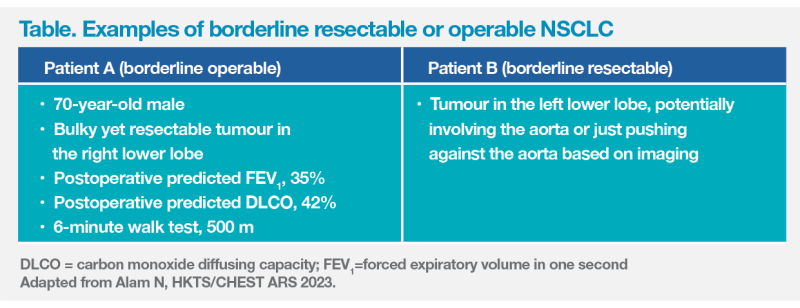
“If an early-stage NSCLC patient has a borderline resectable or borderline operable tumour, should we offer surgery?” questioned Alam. (Table)
“In patients with borderline resectable or operable NSCLC who have a PD-L1 expression TC of ≥50 percent, I would push for upfront surgery [plus adjuvant chemotherapy] and atezolizumab, as their chance of cure is high [with a 5-year OS rate of 84.8 percent],” said Alam. (Figure)
Controversies of neoadjuvant IO
Neoadjuvant IO is a topic of interest in early-stage NSCLC management. The phase III CheckMate 816 trial showed that a substantial proportion of patients (15.6 percent) treated with neoadjuvant nivolumab plus chemotherapy did not undergo definitive surgery due to disease progression, adverse events, patient refusal, being unfit for surgery because of poor lung function, unresectability, or consent withdrawal. Other potential preoperative issues related to neoadjuvant IO included delays in definitive surgery and pneumonitis. [N Engl J Med 2022;386:1973-1985; Transl Lung Cancer Res 2021;10:563-580]
In addition, concerns have arisen among surgeons regarding the inflammatory consequences of neoadjuvant treatment, which is problematic at the time of surgery. “It is counterintuitive,” commented Alam. “Neoadjuvant IO is supposed to reduce operative difficulty due to tumour shrinkage, but it did the opposite in some cases due to fibrosis and inflammation in lymph nodes as well as tissue adhesion.” [Transl Lung Cancer Res 2021;10:563-580; Transl Lung Cancer Res 2020;9:2696-2715]
These inflammatory consequences of neoadjuvant treatment led to longer operative times, higher conversion rates from minimally invasive surgery (MIS) to thoracotomy, and higher complication rates. “In cases of lymph node fibrosis between the pulmonary artery and bronchus, thoracotomy may be required to get around the pulmonary artery,” noted Alam. As demonstrated in CheckMate 816, patients receiving neoadjuvant nivolumab plus chemotherapy had markedly high rates of thoracotomy (59.1 percent) and conversion from MIS to thoracotomy (11.4 percent). Notably, pneumonectomy, which was associated with permanent lung function loss and high rate of perioperative mortality, was required in 16.8 percent of patients. [Transl Lung Cancer Res 2021;10:563-580; N Engl J Med 2022;386:1973-1985]
Summary
Surgeons’ input is important in evaluation of resectability and operability as well as in the choice between neoadjuvant therapy and upfront surgery ± adjuvant therapy. The risk of inflammatory consequences and surgical attrition with neoadjuvant IO should be cautiously considered. In early-stage NSCLC patients with PD-L1 expression TC of ≥50 percent, surgery plus adjuvant atezolizumab is a promising treatment option with proven survival benefits.