Early use of an endothelin receptor antagonist in a patient with scleroderma-spectrum connective tissue disease–associated PAH





Presentation, investigations and treatment
A 39-year-old female hospital porter presented with dyspnoea and Raynaud’s phenomenon in 2010, with no evidence of sclerodactyly. She tested positive for anti-ribonucleoprotein (anti-RNP) and anti–Scl-70 autoantibodies. High-resolution CT (HRCT) of the chest revealed bilateral ground-glass opacities, suggesting interstitial thickening or fibrosis. Echocardiography showed an elevated estimated right ventricular systolic pressure (RVSP) of 74 mm Hg. These findings, along with other defining features, supported a diagnosis of mixed connective tissue disease (MCTD), complicated by interstitial lung disease (ILD) and possibly pulmonary arterial hypertension (PAH).1 (Table)
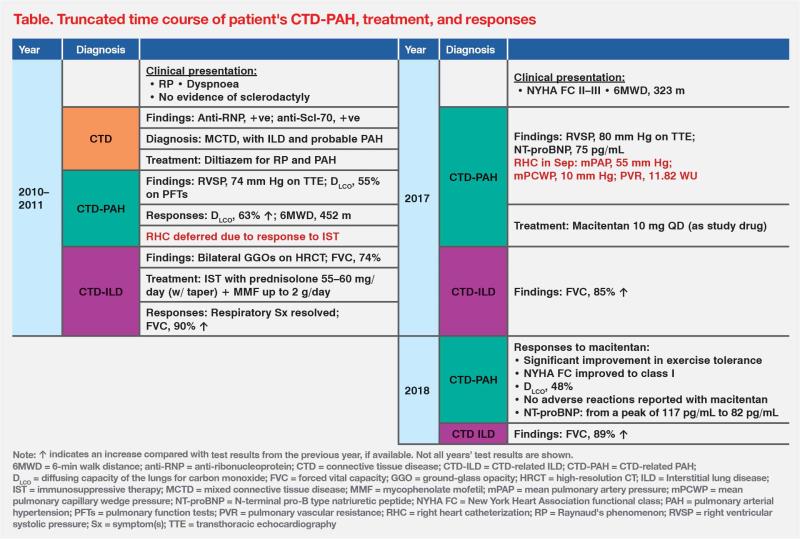
Upon diagnosis, the patient was started on sustained-release diltiazem (100 mg/day) for Raynaud’s phenomenon and possible PAH. She also received prednisolone (55–60 mg/day) that year, followed by mycophenolate mofetil (MMF; up to 2 g/day) in 2011 for ILD. Subsequently, her respiratory symptoms resolved. Repeat echocardiography showed decreased RVSP of 47 mm Hg, obviating the need for right heart catheterization (RHC). (Table)
In 2016, the patient once again experienced dyspnoea on exertion, but ILD progression was ruled out by HRCT. Pulmonary function tests (PFTs) detected a reduced haemoglobin-corrected lung diffusion capacity for carbon monoxide (DLCO) of 47 percent. Serial echocardiography revealed a rising trend in RVSP over the years, peaking at 80 mm Hg. RHC was thus performed in September 2017 to confirm a diagnosis of PAH, revealing an elevated mean pulmonary artery pressure (mPAP) of 55 mm Hg and a heightened pulmonary vascular resistance of 11.82 WU. The patient's respiratory symptoms had a negative impact on her daily activities and work, limiting her to being able to walk up only one flight of stairs (New York Heart Association [NYHA] functional class, II–III). (Table)
A month later, the patient was enrolled in a local clinical trial and received the endothelin receptor antagonist (ERA), macitentan (10 mg QD). After a few months of treatment, the patient reported a significant improvement in exercise tolerance and was able to climb three flights of stairs. Her symptoms eventually improved to NYHA functional class I within 1 year of macitentan treatment. (Table)
In 2020, the patient’s MCTD progressed to systemic sclerosis (SSc), as evidenced by development of predominant scleroderma features over time.2 Following this, her dyspnoea worsened, necessitating repeat RHC to assess if her PAH had progressed, which was ruled out with mPAP of 36 mm Hg. Instead, HRCT in late 2020 revealed worsening of lung fibrosis and development of interstitial pneumonia. This prompted an increase in dosages of prednisolone and MMF, alongside initiation of a tyrosine kinase inhibitor, nintedanib. Her respiratory status subsequently improved.
In 2021, a phosphodiesterase 5 inhibitor (PDE5i), sildenafil citrate, was added as a double combination with macitentan in view of accumulating clinical evidence. As of the latest follow-up in January 2024, the patient, at 53 years of age, was still on the ERA/PDE5i combination and remained active despite physical demands of her job. She could walk unaided and had no complaints of dyspnoea, placing her in NYHA functional class I–II.
Discussion
CTDs are a group of heterogeneous diseases (eg, MCTD, SSc) that cause a myriad of pulmonary complications, including PAH and ILD in up to 15 percent and 40 percent of patients, respectively.3 As observed in our patient, dyspnoea can be attributed to either ILD or PAH during the first 10-year course of her disease.
CTD-PAH is the second most common type of PAH after idiopathic PAH (iPAH). The REVEAL registry showed that patients with CTD-PAH had twice the risk of 1-year mortality vs iPAH patients (14 percent vs 7 percent). Among CTD-PAH subtypes, SSc is the most common and is associated with the worst survival (1-year mortality rate, 18 percent).4 Regardless of CTD-PAH subtype, PAH-related right heart failure is the primary cause of death.5
As early detection of PAH in CTD patients may lead to better survival outcomes, the 2022 European Society of Cardiology (ESC)/European Respiratory Society (ERS) pulmonary hypertension guidelines recommend annual evaluation of PAH risk in patients with SSc, and suggest considering PAH risk evaluation for other CTDs with overlapping scleroderma features.3,6
The ESC/ERS guidelines gave a class I recommendation to the 2-step DETECT algorithm for PAH screening, which includes nonechocardiographic variables (eg, DLCO, N-terminal pro-B type natriuretic peptide [NT-proBNP]) and echocardiography.6 Alternatively, the Australian Scleroderma Interest Group (ASIG) algorithm can be easily implemented, where only two noninvasive parameters (ie, NT-proBNP level and pulmonary function test) serve as a “first-tier” test (sensitivity, 100 percent; specificity, 77.8 percent).7
Following confirmed PAH diagnosis, comprehensive risk assessment should be conducted to inform prognosis and management choices. In the ESC/ERS guidelines, initial combination with ERA and PDE5i were suggested in patients with low or intermediate risk. Addition of prostacyclin receptor agonist can be considered if patients failed to respond to initial therapy, following the results from GRIPHON study that selexipag lowered composite endpoint of death and PAH complication. Prostacyclin analogue is reserved for selected patients with high risk.6,8
Since CTD-PAH is a lifelong disorder that progresses over time, long-term survival outcomes of PAH-specific therapy can help inform clinical practice. SERAPHIN OL, an openlabel extension of the SERAPHIN of macitentan, provided insights into longterm safety of macitentan 10mg in PAH patients.9 Notably, 30.3 percent of patients randomized to the macitentan 10 mg arm in SERAPHIN were classified as CTD-PAH, of whom 52.1 percent had SSc-PAH.10 After 9 years of follow-up for the overall population in the macitentan 10 mg arm (n=242), survival rate was 52.7 percent, while long-term safety was comparable to the known safety profile of macitentan.9
Early referral to a cardiologist for consideration of RHC and multidisciplinary care in PAH are needed. The above cases illustrated the long-term benefit following early treatment with macitentan-based combination therapy in a patient with CTD-PAH. In Hong Kong’s public healthcare system, starting treatment with a PDE5i and adding ERA after confirming PAH with RHC may be a sensible approach.