Extended phase III results support nivolumab as adjuvant SoC in MIBC
Technical University of Munich
Munich, Germany

Neoadjuvant systemic therapy plus radical cystectomy is the current standard of care (SoC) for patients with muscle-invasive urothelial carcinoma (MIUC), including muscle-invasive bladder carcinoma (MIBC). However, patients with MIUC remain at high risk of lethal metastatic recurrence. At the 28th Annual Scientific Meeting of the Hong Kong Urological Association, Professor Jürgen Gschwend of the Technical University of Munich in Munich, Germany, discussed extended follow-up results of the CheckMate 274 trial, which demonstrated continued survival benefits with adjuvant nivolumab in patients with high-risk MIBC.
Current trends in MIBC management
“Major guidelines, such as the European Association of Urology [EAU] and National Comprehensive Cancer Network [NCCN], recommend neoadjuvant systemic therapy followed by radical cystectomy as SoC for patients with MIBC,” said Gschwend. “Cisplatin-based combination chemotherapy is the preferred preoperative regimen for cisplatin-fit patients with MIBC [T2–T4a, N0M0 disease].” [Eur Urol 2023;doi:10.1016/j.eururo.2023.08.016; NCCN Clinical Practice Guidelines in Oncology, Bladder Cancer, version 3.2023; Oncologist 2016;21:708-715]
“Since systemic therapy and surgery are part of the SoC in patients with MIBC, a multidisciplinary team is crucial to ensure optimal communication between urologists and surgeons so that patients are provided with best treatment options,” he opined.
CheckMate 274: Adjuvant nivolumab as SoC
Patients with MIUC are at high risk of lethal metastatic recurrence, with most recurrences occurring within 2–3 years after surgery. [Front Oncol 2015;5:246; Milowsky MI, et al, AUA 2023, abstract LBA02-08]
Of studies investigating adjuvant immunotherapy in MIUC, CheckMate 274 was the first positive phase III trial demonstrating a significant disease-free survival (DFS) benefit with an immune checkpoint inhibitor (ie, nivolumab) vs placebo in high-risk MIUC after radical surgery. [N Engl J Med 2021;384:2102-2114] The DFS benefit was sustained after a minimum of 31.6 months of follow-up. [Galsky MD, et al, ASCO GU 2023, abstract LBA443]
“CheckMate 274 was a real game-changer study,” remarked Gschwend. Patients in the multicentre, randomized, double-blind CheckMate 274 trial had radical surgery, with or without neoadjuvant cisplatin-based chemotherapy. They had pathological evidence of urothelial carcinoma originating in the bladder, ureter or renal pelvis, with a high risk of recurrence. Patients with MIBC (the most predominant type of urothelial carcinoma) comprised 79 percent of the intent-to-treat (ITT) population. Nivolumab 240 mg or placebo was administered Q2W as a 30-minute intravenous infusion for ≤1 year or until disease recurrence or trial discontinuation. Primary endpoints were DFS in the ITT population and in patients with tumour PD-L1 expression level of ≥1 percent. [N Engl J Med 2021;384:2102-2114]
Results
In the ITT population, nivolumab (n=353) was associated with a longer median DFS vs placebo (n=356) (20.8 months vs 10.8 months). Among patients with PD-L1 ≥1 percent, the corresponding DFS rates at 6 months were 74.5 percent vs 55.7 percent (hazard ratio [HR], 0.55; 98.72 percent confidence interval [CI], 0.35–0.85; p<0.001). Nivolumab was consistently associated with DFS benefit vs placebo regardless of nodal status, PD-L1 status or previous neoadjuvant cisplatin-based chemotherapy. [N Engl J Med 2021;384:2102-2114]
In a subgroup analysis of patients with MIBC, who comprised nearly 80 percent of the overall population, the 12-month DFS rate was 66 percent with nivolumab (n=279) and 45 percent with placebo (n=281) (HR, 0.61; 95 percent CI, 0.49–0.77). DFS was improved with nivolumab vs placebo across subgroups according to age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS), nodal status, and PD-L1 expression status. [Witjes A, et al, ASCO 2022, abstract 4585]
“The DFS benefit was especially pronounced in patients with PD-L1 ≥1 percent [HR, 0.46; 95 percent CI, 0.31–0.67], but it was still significant in MIBC patients with negative PD-L1 expression [<1 percent; HR, 0.70; 95 percent CI, 0.53–0.94], suggesting that PD-L1 expression does not play a very big role in bladder cancer,” commented Gschwend.
The safety profile of nivolumab was consistent with previous trials in patients advanced UC and other cancers, with no new safety signals reported. Treatment-related adverse events (TRAEs) of grade ≥3 occurred in 17.9 percent vs 7.2 percent of patients in the nivolumab vs placebo group. In the ITT population, 53.3 percent vs 56.3 of patients on nivolumab vs placebo discontinued the trial regimen. [N Engl J Med 2021;384:2102-2114]
Extended follow-up results
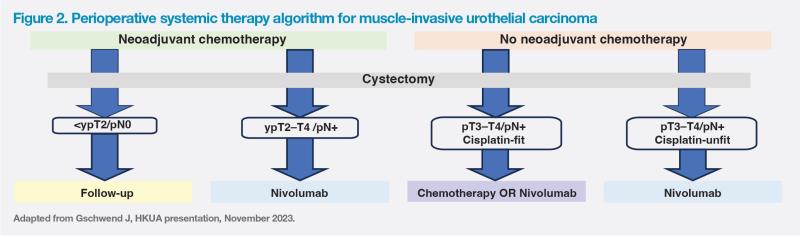
After a minimum follow-up of 31.6 months, median DFS was 22.0 months with nivolumab vs 10.9 months with placebo in the ITT population (HR, 0.71; 95 percent CI, 0.58–0.86). In the MIBC population, nivolumab was associated with a three-fold improvement in DFS vs placebo (median, 25.6 months vs 8.5 months; HR, 0.63; 95 percent CI, 0.51–0.78). (Figure 1)
While overall survival results are not yet mature, second progression-free survival (PFS2) was improved with nivolumab vs placebo in both ITT (HR, 0.79; 95 percent CI, 0.63–0.98) and PD-L1 ≥1 percent (HR, 0.54; 95 percent CI, 0.37–0.79) populations.
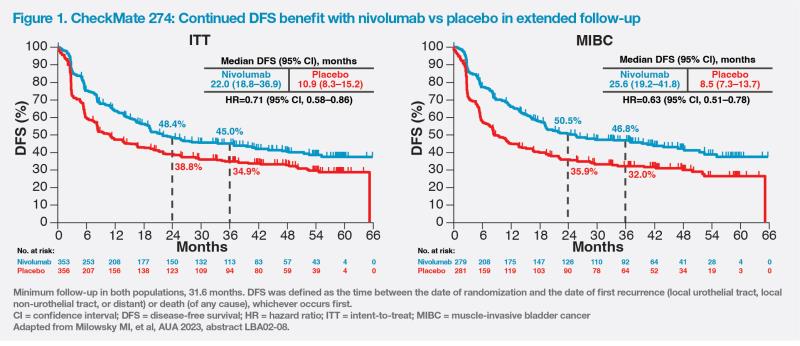
“On the basis of extended follow-up results, adjuvant immunotherapy with nivolumab should be considered a SoC in MIUC after radical resection,” concluded Gschwend. (Figure 2)