First-line immunotherapy plus chemotherapy in metastatic biliary tract cancer








 HIMALAYA trial showed that complete and partial responses are considerably more likely in patients with unresectable HCC treated with immunotherapy
vs the previous standard of care, sorafenib.
HIMALAYA trial showed that complete and partial responses are considerably more likely in patients with unresectable HCC treated with immunotherapy
vs the previous standard of care, sorafenib.Case 1: Ongoing response with 23 cycles of durvalumab
A 69-year-old female who enjoyed good past health presented with a 3-month history of right-sided abdominal discomfort in December 2022.
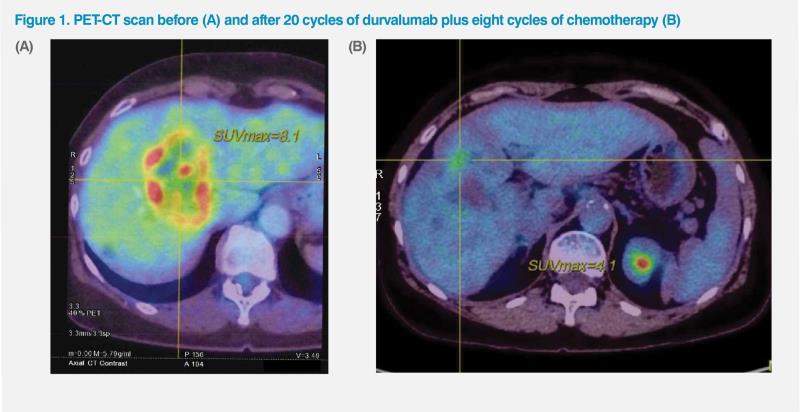
A PET-CT scan in the same month revealed multiple hypodense masses in the liver, which appeared matted together and had elevated FDG uptake. The most prominent mass in the dome of the liver measured 5.79 x 5.75 cm, with SUVmax of 8.1. (Figure 1A) Focal eccentric wall thickening was noted over the body and fundus of the gallbladder, measuring 1.80 x 1.86 cm, with associated elevated FDG uptake (SUVmax, 9.6). Enlarged lymph node (LN) was noted at the supraclavicular fossa, along with a lung nodule in the right apex. An enlarged LN was also noted over porta hepatis.
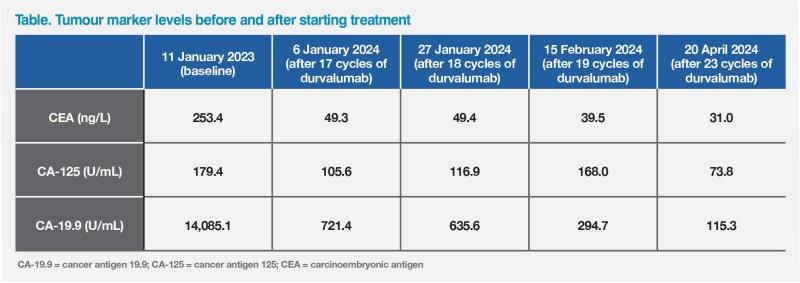
Laboratory investigations in January 2023 showed elevated levels of carcinoembryonic antigen (CEA; 243.4 ng/mL), cancer antigen 125 (CA-125; 179.4 U/mL) and cancer antigen 19.9 (CA-19.9; 14,085.1 U/mL) tumour markers. Ultrasound-guided biopsy indicated adenocarcinoma, but results of immunohistochemistry were inconclusive. The patient was diagnosed with metastatic gallbladder tumour on the basis of PET-CT imaging and by exclusion of alternative tumour origins in view of negative markers for lung, colorectal, gynaecological, and renal cancers.
Extensive next-generation sequencing (NGS) did not reveal any targetable mutations. The tumour was found to be mismatch repair (MMR)–proficient, and no microsatellite instability (MSI) was identified. The patient was recommended combination therapy with durvalumab (1,500 mg, day 1 of 21-day cycle) plus gemcitabine (1,000 mg/m2, days 1 and 8 of 21-day cycle) plus platinum-based chemotherapy, which she commenced in January 2023, 1 week after diagnosis.
The patient received a total of eight chemotherapy cycles and, as of April 2024, 23 cycles of durvalumab. Her abdominal discomfort resolved after the first treatment cycle, and her Eastern Cooperative Oncology Group performance status (ECOG PS) remained 0 since presentation and throughout treatment.
A repeat PET-CT scan in February 2024 indicated excellent partial response to treatment. No abnormal FDG uptake was noted in the gallbladder. A residual hypodense lesion in medial segment of the left liver lobe had decreased in size (2.25 x 1.88 cm) and showed lower FDG uptake (SUVmax, 4.1) compared with pretreatment PETCT scan. (Figure 1B) Other hypodense liver lesions in the right liver lobe had no abnormal FDG uptake, suggesting successfully treated liver metastases. Overall, the patient achieved excellent partial response.
The patient’s tumour marker levels have been in decline over 16 months of treatment. (Table)
During the chemotherapy phase of treatment, the patient experienced asymptomatic grade 1 neutropenia and grade 1 thrombocytopenia, which were managed with a granulocyte colony–stimulating factor and eltrombopag, respectively. No other adverse events (AEs) were noted.
Treatment with durvalumab is expected to continue for a total of 2 years.
Case 2: Early response to durvalumab + chemotherapy
A 40-year-old male presented with weight loss, tarry stools, abdominal distension, and epigastric pain in July 2023. He had hypertension and drank beer socially.
Esophagogastroduodenoscopy clearly showed extrinsic compression. Helicobacter pylori test was negative. A follow-up CT scan revealed hepatomegaly caused by a very large bilobar liver mass. A subsequent PET-CT scan on 10 July 2023 confirmed these findings, showing a hypermetabolic (SUVmax, 12.4) mass measuring 13.6 x 10.9 x 17.3 cm, predominately located in the right lobe, but also multiple other foci in the bilateral lobes of the liver. In addition, multiple LNs appeared to be involved, including retrocaval, aortocaval, para-aortic, and celiac areas. The largest LN appeared in supraclavicular area and measured 1 cm. Ascites was also noted. Patient’s ECOG PS was 1 at this time.
Laboratory tests showed normal levels of alfa-fetoprotein (AFP) and CEA tumour markers, while CA-19.9 level was elevated at 181 U/mL. Differential diagnosis of cholangiocarcinoma or hepatocellular carcinoma (HCC) was proposed.
Liver biopsy results were consistent with adenocarcinoma. While immunostaining results were inconclusive as to the primary site, they excluded possibility of HCC, leading to diagnosis of cholangiocarcinoma by exclusion. As liver biopsy produced an adequate tissue sample, NGS testing was suggested to the patient, but he refused due to financial concerns.
Following a discussion with the patient, treatment with durvalumab plus gemcitabine plus cisplatin (GC) was commenced in late July 2023.
The patient tolerated treatment very well. Full doses of immunotherapy and chemotherapy were administered on days 1 and 8 of each 21-day cycle on schedule without delay. The patient began to feel better after the first cycle of treatment, and his condition improved considerably after two cycles, when he reported a resolution of epigastric pain and a reduction in abdominal distension.
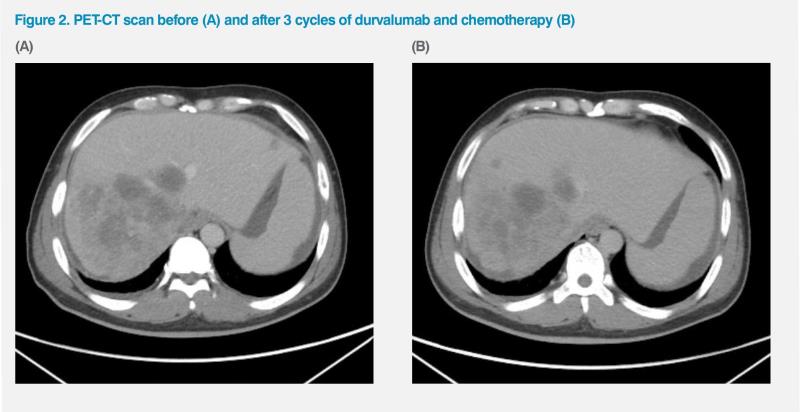
An interim PET-CT scan in September 2023, after three treatment cycles, showed an improvement in LNs and a reduction in dimensions (longest, 12.5 cm) and hypermetabolic activity (SUVmax, 10.1) in the large liver lesion, while some ascites remained. (Figure 2) His CA-19.9 was 212 U/mL on 15 August 2023, dropping to 153 U/mL on 5 September 2023, to 96 U/mL on 26 September, to 74 U/mL on 17 October, to 58 U/mL on 7 November, to 57 U/mL on 28 November, and 43 U/mL on 20 December, over the course of eight cycles.
Regrettably, the patient’s condition began to deteriorate in January 2024, after completion of eight cycles of immuno-chemotherapy. A follow-up PET-CT in the same month showed new liver lesions and increasing ascites. To avoid prolonged inpatient stays required for the administration of FOLFOX (fluorouracil plus oxaliplatin) infusions, the patient began second-line treatment with XELOX (capecitabine plus oxaliplatin) in February 2024, but did not respond well after two cycles. When the patient was last seen in April 2024, his ascites required regular draining, yielding several litres of fluid every time. He began to suffer from haematemesis and cachexia and lost approximately 15 kg in the past 2 months. His ECOG PS was 2.
The patient eventually agreed to NGS testing, which showed IDH1 mutation. He is currently considering third-line therapy or hospice care.
Discussion
Biliary tract cancer (BTC) is a heterogeneous group of malignancies consisting of intrahepatic cholangiocarcinoma (iCCA), extrahepatic cholangiocarcinoma (eCCA), and gallbladder adenocarcinoma (GBA).1 It is typically diagnosed at advanced stages, when curative surgery is not feasible, leading to poor prognosis.2 Despite several trials evaluating potential use of targeted therapy, GC chemotherapy has remained the first-line standard of care (SoC) for advanced BTC for over a decade. It is associated with a median overall survival (OS) of 11.2 months and a 1-year OS rate of 39 percent. Surveillance, Epidemiology, and End Results (SEER) data collected for patients diagnosed with metastatic BTC between 2012 and 2018 indicate a 5-year relative survival rate of 3 percent.3,4
Early-phase studies demonstrated clinical activity of immune checkpoint inhibitors, including durvalumab (a PD-L1 inhibitor), in BTC.5-7 A phase II trial of durvalumab in combination with GC demonstrated promising efficacy, with an objective response rate (ORR) of 72 percent and a median OS of 20.2 months, without dose-limiting toxicity.8 The subsequent randomized, double-blind, placebo-controlled, global TOPAZ-1 trial became the first phase III study to confirm that adding immunotherapy to SoC chemotherapy in the first line delivers a significant OS benefit to patients with locally advanced or metastatic BTC.
In TOPAZ-1, 685 previously untreated patients with unresectable, metastatic or recurrent BTC were randomized to receive durvalumab (n=341) or placebo (n=344) in combination with GC for up to eight cycles, followed by durvalumab or placebo monotherapy until disease progression or unacceptable toxicity. OS was the primary endpoint, while progression-free survival (PFS), ORR and safety served as secondary endpoints.8
At preplanned interim analysis, with a median follow-up of 16.8 months for durvalumab and 15.9 months for placebo, OS was significantly longer with durvalumab vs placebo (12.8 vs 11.5 months; hazard ratio [HR], 0.80; 95 percent confidence interval [CI], 0.66–0.97; p=0.021). Estimated OS rates were 54.1 vs 48.0 percent at 12 months, 35.1 vs 25.6 percent at 18 months, and 24.9 vs 10.4 percent at 24 months, respectively.8
Of note, after 6 months, there was a clear and sustained separation of the OS Kaplan–Meier curves in favour of durvalumab. While the OS HR was 0.91 (95 percent CI, 0.66–1.26) up to 6 months, it reduced to 0.74 (95 percent CI, 0.58–0.94) after 6 months.8
Median PFS was 7.2 vs 5.7 months with durvalumab vs placebo (HR, 0.75; 95 percent CI, 0.63–0.89; p=0.001). A trend towards OS and PFS benefit with durvalumab plus chemotherapy was observed across all subgroups analyzed. The addition of durvalumab to chemotherapy benefited patients regardless of PD-L1 expression level, indicating that PD-L1 status may be of limited value in predicting durvalumab’s clinical benefit in this patient population. 8 As a result, PD-L1 testing is not routinely carried out before prescribing durvalumab to BTC patients in Hong Kong and was not done for either of our patients.
Investigator-assessed ORR was 26.7 vs 18.7 percent in the durvalumab vs placebo group (odds ratio [OR], 1.60; 95 percent CI, 1.11–2.31). Continued response was sustained for ≥12 months in 26.1 vs 15.0 percent of patients.8 Notably, the patient in case 1 remained in response 16 months after commencing treatment with durvalumab plus chemotherapy.
Median time to response was 1.6 vs 2.7 months in patients treated with durvalumab vs placebo.8 These findings were echoed in case 2, when the patient experienced early response to treatment, which manifested as symptomatic improvement after just one cycle.
Grade 3/4 AEs occurred in 75.7 and 77.8 percent of patients in the durvalumab and placebo groups, respectively.
The respective rates of discontinuation of any treatment component due to AEs were 13.0 and 15.2 percent, while deaths due to AEs occurred in 3.6 and 4.1 percent of patients. The most common AEs of any grade were anaemia (48.2 percent), nausea (40.2 percent), constipation (32.0 percent), and neutropenia (31.7 percent) in the durvalumab group and anaemia (44.7 percent), nausea (34.2 percent), and neutropenia (31.0 percent) in the placebo group. Rates of immune-mediated AEs were 12.7 vs 4.7 percent with durvalumab vs placebo.8 Durvalumab was not found to contribute additional toxicity to that observed with chemotherapy, which is consistent with our patients’ experience.
Based on these interim results, the National Comprehensive Cancer Network (NCCN) included durvalumab plus GC as a category 1 preferred regimen for first-line systemic treatment of unresectable or metastatic BTC.9
At median follow-up of 23.4 and 22.4 months in the durvalumab and placebo groups, the respective median OS was 12.9 vs 11.3 months (HR, 0.76; 95 percent CI, 0.64–0.91). As observed in the primary analysis, the OS HR reduced after 6 months and became 0.71 (95 percent CI, 0.58–0.88).10 The OS benefit was observed across all prespecified subgroups, regardless of disease status at baseline (initially unresectable or recurrent disease), region (Asia or rest of the world), primary tumour location (iCCA or eCCA or GBA) and diagnostic stage (locally advanced or metastatic disease).10
Durvalumab plus GC continued to demonstrate consistent, clinically meaningful and durable benefits in patients with advanced BTC in the updated, 3-year analysis of the TOPAZ-1 trial. With median follow-up of 42.9 and 41.8 months in the durvalumab and placebo groups, respectively, the OS rate of patients alive at 36 months was 14.6 percent in the durvalumab plus GC arm and 6.9 percent in the placebo plus GC arm.11
Key takeaways
TOPAZ-1 is the first phase III trial to demonstrate that addition of immunotherapy to previous SoC of GC produces a significant OS benefit in metastatic or advanced BTC. Addition of durvalumab to chemotherapy was beneficial across all prespecified subgroups in TOPAZ-1, meaning that it is suitable for BTC patients regardless of their primary tumour location. Both of our patients, one of whom had CCA and the other GBA, had early response to treatment and experienced symptom improvement after just one cycle of immuno-chemotherapy, while the patient with metastatic GBA remained in response 16 months after commencing treatment.
In summary, based on clinical experience and the results of the TOPAZ-1 trial, even metastatic BTC patients should not be denied the chance of extended survival with durvalumab plus GC.