First-line PARP inhibitor in a patient with stage III homologous recombination proficient ovarian cancer
Specialist in Obstetrics and Gynaecology
Queen Mary Hospital
Hong Kong

History and presentation
A 45-year-old female patient with good past health and a body weight of 53 kg presented with abdominal distension of 2 months. Physical examination showed gross ascites. Abdominal tapping was performed and cytology showed atypical cells in the ascitic fluid.
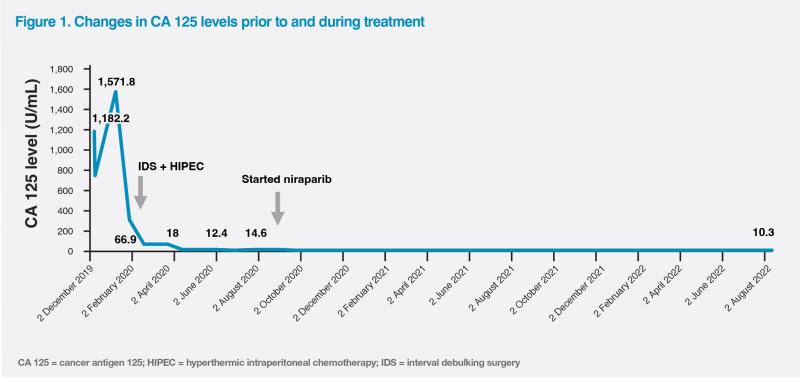
CT scan of the thorax, abdomen and pelvis on 18 November 2019 revealed a complex solid and cystic left adnexal mass (5 cm x 5 cm x 7 cm), gross ascites and substantial subdiaphragmatic peritoneal disease. There were multiple enhancing peritoneal masses in the pouch of Douglas, bilateral subphrenic space, bilateral paracolic gutter and around the caudate lobe and omentum, with the largest lesion measuring 6 cm. Cancer antigen 125 (CA 125) was markedly elevated to 1,182 U/mL. (Figure 1)
Diagnostic laparoscopy, performed in November 2019 to assess feasibility of upfront surgery, showed extensive peritoneal carcinomatosis, omental cake and 10 cm bilateral ovarian tumours. Her Fagotti score was 8 due to the presence of omental cake, peritoneal carcinosis, diaphragmatic carcinosis and mesenteric retraction (2 points for each).1 Findings were consistent with a diagnosis of International Federation of Gynaecology and Obstetrics (FIGO) stage IIIc ovarian cancer. Biopsy taken at the time of diagnostic laparoscopy showed high-grade serous carcinoma (HGSC).
Treatment and response
In view of the extensive disease, complete cytoreduction was deemed not possible, and therefore, the patient was advised to have three cycles of neoadjuvant chemotherapy (NACT) from November 2019 to January 2020, followed by reassessment of response and operability.
The patient achieved a good partial response with NACT (left adnexal mass shrinking to 3 cm x 3 cm x 4 cm and a few <1 cm peritoneal nodules). Interval debulking surgery (IDS) plus hyperthermic intraperitoneal chemotherapy (HIPEC) was performed on 28 February 2020, which led to complete debulking with no residual disease (R0). She was then given six more cycles of chemotherapy with paclitaxel and carboplatin between March and August 2020, which resulted in a complete response (CR), with CA 125 level normalizing to 18 U/mL in April 2020. (Figure 1)
The patient refused germline BRCA testing. Homologous recombination deficiency (HRD) testing revealed homologous recombination proficient (HRP) status. Because of that, she was started on maintenance niraparib with an individualized starting dose of 200 mg daily on 3 September 2020.2
During the first 2 weeks of niraparib treatment, the patient experienced grade 3 thrombocytopenia (platelet count, 46 x 109/L). Niraparib was temporarily withheld on 17 September 2020. Her thrombocytopenia resolved after 2 weeks, following which niraparib was restarted at 100 mg daily.2
The patient responded well to niraparib maintenance, with CA 125 levels remaining normal in August 2022. (Figure 1) Her Eastern Cooperative Oncology Group (ECOG) performance status remained 0 throughout niraparib treatment.
In September 2022, a borderline low platelet count (70 x 109/L) was noted. Niraparib was discontinued – 25 months since treatment initiation. Her platelet counts remained static at approximately 100 x 109/L since then. Last seen in September 2023, the patient remained well with no evidence of recurrence.
Discussion
R0 resection plays a crucial role in improving overall survival (OS) and progression-free survival (PFS) in advanced ovarian cancer.3 Diagnostic laparoscopy is a useful tool for assessment of resectability in patients with preoperative imaging showing extensive intra-abdominal disease.1,3 Biopsy could also be obtained at the time of laparoscopy for histology and further molecular testing.1,4 Different scoring systems have been reported for prediction of complete debulking at the time of the operation.
One of the examples is Fagotti score –a score of ≥8 indicates a low likelihood of achieving R0 resection.1 In these patients, NACT with carboplatin and paclitaxel for 3–6 cycles followed by IDS and further adjuvant chemotherapy would be recommended. Our patient who initially had unresectable disease achieved good partial response with NACT, and R0 resection was achieved at the time of IDS.
Careful consideration is warranted before prescribing bevacizumab in patients with bowel involvement, due to an increased risk of bowel perforation (15.4 percent for ovarian cancer patients).5 At our centre, bevacizumab is usually withheld ≥4 weeks before surgery to reduce the risk of wound-healing complications, and therefore bevacizumab is usually withheld in the last cycle of NACT.
Both poly(ADP-ribose) polymerase (PARP) inhibitors and bevacizumab are available as maintenance therapy for newly diagnosed advanced ovarian cancer patients.6 As biomarkers (ie, BRCA mutations, HRD status) are crucial for treatment selection and prognosis prediction, genetic testing should be considered for all patients with advanced ovarian cancer.7,8 Obtaining tumour tissue before initiation of NACT is important since there is a possibility that tumour tissue would not be available for further molecular testing after chemotherapy.8
According to the American Society of Clinical Oncology (ASCO) guidelines, women with newly diagnosed stage III–IV epithelial ovarian, tubal, or primary peritoneal cancer that is in complete or partial response to first-line platinum-based chemotherapy should be offered PARP inhibitor maintenance therapy with olaparib (for those with germline or somatic pathogenic or likely pathogenic variants in BRCA1/2 genes) or niraparib (all women) in high-grade serous or endometrioid ovarian cancer (evidence quality, high; strength of recommendation, strong), including those with HRP tumours, such as our patient.9
The efficacy and safety of niraparib maintenance therapy in newly diagnosed advanced ovarian cancer were demonstrated in the randomized, double-blind, placebo-controlled phase III PRIME study (n=384), which included FIGO stage III–IV patients who had undergone primary debulking surgery (PDS) or IDS, irrespective of postoperative residual disease status. All patients in PRIME were Chinese, with individual starting dose of niraparib used prospectively, making the findings particularly relevant to Hong Kong’s patient population. Most patients in PRIME had FIGO stage III disease (71.4–72.9 percent), achieved CR with platinum-based chemotherapy (79.8–83.1 percent), and had optimal cytoreduction (R0/R1; 75.7–81.4 percent).10
At a median follow-up of 27.5 months, niraparib monotherapy significantly prolonged median PFS by 16.5 months vs placebo (24.8 months vs 8.3 months; hazard ratio [HR], 0.45; 95 percent confidence interval [CI], 0.34–0.60; p<0.001) in the intention-to-treat population. The PFS benefit of niraparib was seen in all comers, regardless of BRCA or HRD status.10
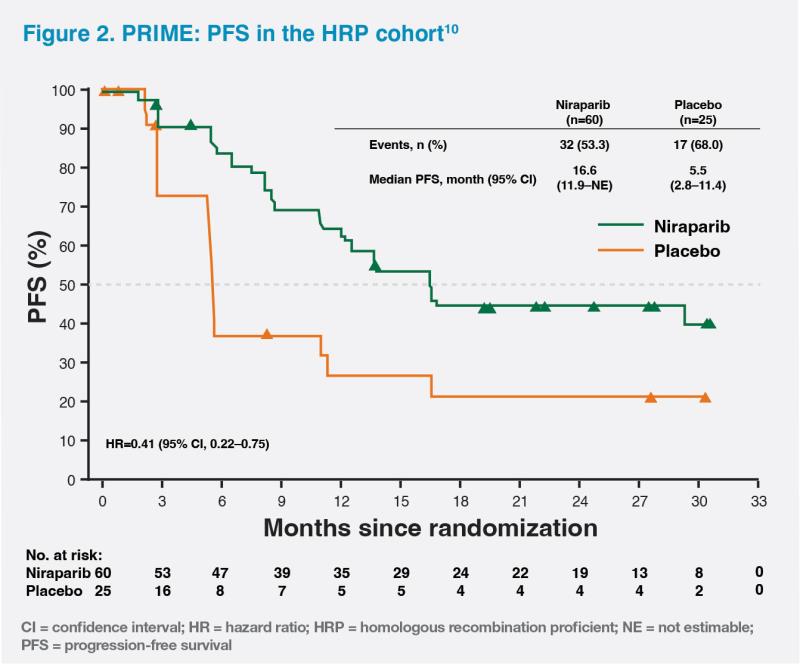
HRP status, as in the case of our patient, is associated with the worst prognosis.11 In the HRP cohort of PRIME, niraparib monotherapy significantly reduced the risk of disease progression or death by 59 percent vs placebo (median PFS, 16.6 months vs 5.5 months; HR, 0.41; 95 percent CI, 0.22–0.75). The PFS curves separated at 3 months and remained separated throughout the study, demonstrating a prolonged effect of niraparib on PFS in the HRP subgroup. (Figure 2) Our patient’s PFS was 3 years – at least twice as long as the median PFS in the HRP cohort of the PRIME trial.10 Based on the data from PRIME, niraparib should be considered in all HRP patients.
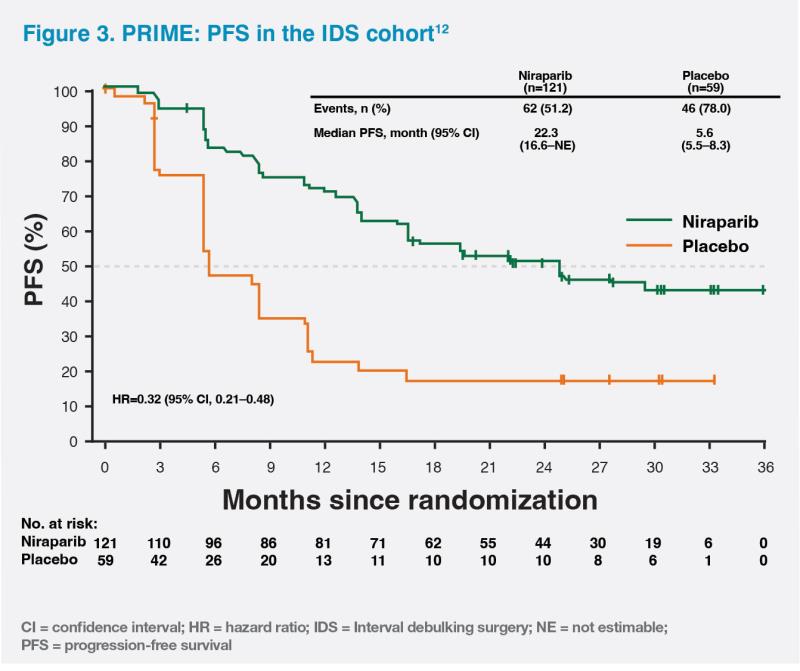
Furthermore, niraparib maintenance significantly improved median PFS vs placebo regardless of postoperative residual disease status (R0/R1 resection: 24.8 months vs 8.3 months; HR, 0.44; 95 percent CI, 0.32–0.61) (R2 resection: 16.5 months vs 8.3 months; HR, 0.27; 95 percent CI, 0.10–0.72) or surgical timing (IDS: 22.3 months vs 5.6 months; HR, 0.32; 95 percent CI, 0.21–0.48) (PDS: not reached vs 12.0 month; HR, 0.63; 95 percent CI, 0.42–0.94), providing improved outcomes for a broad range of ovarian cancer patients.10,12 (Figure 3)
Positive results from PRIME supplement the phase III PRIMA study, which excluded patients with stage III disease who had undergone R0 resection at PDS and used an individualized starting dose in around 35 percent of patients only.10
In PRIME, niraparib’s common grade ≥3 haematological treatment-emergent adverse events (TEAEs) included anaemia (18.0 percent), decreased neutrophil count (17.3 percent), decreased platelet count (14.1 percent), and decreased white blood cell count (6.7 percent).10 As demonstrated in our patient’s case, haematological TEAEs are generally transient, occur in the first month, and resolve after niraparib dose interruption. Once the haematological AE is resolved, niraparib can be resumed at a reduced dose (except in patients with first occurrence of thrombocytopenia with platelet count between 75 x 109/L and <100 x 109/L, in whom the same dose of niraparib could be resumed). Close monitoring of complete blood counts (CBCs) is important, especially when initiating and resuming niraparib treatment, during which weekly CBC monitoring is recommended.2
In summary, R0 resection is key in ovarian cancer management.3 Genetic testing should be discussed with all advanced ovarian cancer patients, preferably at the time of initial diagnosis.7,8 In PRIME, first-line niraparib maintenance offers statistically significant PFS benefits to all comers, regardless of postoperative residual disease status, surgical timing and biomarkers status (eg, HRP tumours).10,12