
History and presentation
A 66-year-old male was referred by his family physician in February 2019. At presentation, he was asymptomatic apart from occasional night sweats (once every 2 months). He had a history of hypertension that was well controlled with calcium channel blockers. His Eastern Cooperative Oncology Group performance status (ECOG PS) was 0.
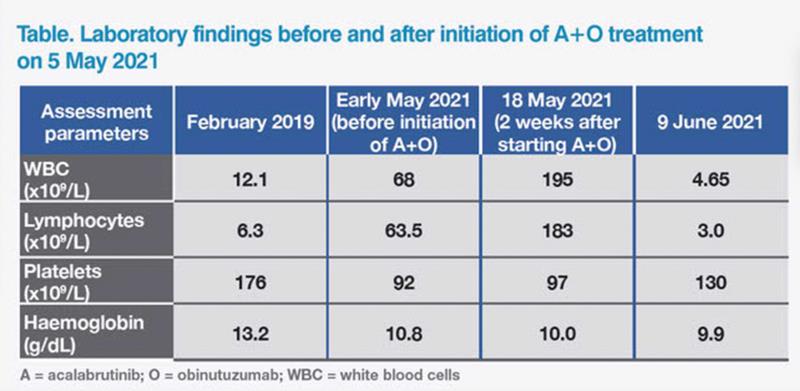
Initial blood test before referral revealed leukocytosis and a borderline low level of haemoglobin, although the patient showed no symptoms of anaemia. (Table) Peripheral blood morphology was compatible with chronic lymphocytic leukaemia (CLL), which was subsequently confirmed by flow cytometry. Fluorescence in-situ hybridization (FISH) analysis detected 13q14.3 deletion. He also carried mutated immunoglobulin heavy-chain variable (IGHV) gene, but did not have del(17p) or TP53 mutation. Physical examination revealed multiple palpable lymph nodes (LNs; measuring 1–2 cm) in the neck. Whole-body CT scan also found multiple lymphadenopathies, all <3 cm, suggestive of nonbulky disease. No hepatosplenomegaly was observed. His CLL was classified as stage IA disease based on the Rai and Binet staging systems.
Since the patient was asymptomatic apart from infrequent night sweats and did not have disease-related complications, a watchful waiting approach was taken, with regular blood monitoring every 3 months.
Treatment and response
In early May 2021, there was evidence of progressive cytopenia, increased lymphadenopathy, hepatosplenomegaly, more frequent night sweats, and weight loss of about 10 lb. On 5 May 2021, the patient started combination therapy with acalabrutinib (a Bruton tyrosine kinase [BTK] inhibitor) and obinutuzumab (an anti-CD20 monoclonal antibody) (A+O) in 28-day cycles. Acalabrutinib was given orally at 100 mg BID in cycle 1, then combined for six cycles with intravenous obinutuzumab (on days 1 [100 mg], 2 [900 mg], 8 [1,000 mg] and 15 [1,000 mg] of cycle 2 and on day 1 [1,000 mg] of cycles 3–7).1 The patient experienced no infusion-related reactions and was followed up weekly thereafter.
Blood test on 18 May 2021 found elevated white blood cell (WBC) count, which is a known transient effect of BTK inhibitors in CLL.2 The patient was asymptomatic, and all palpable LNs had reduced in size. On 29 May 2021, he developed a small (2 cm) buccal haematoma on the left side, which resolved spontaneously within a week. On 9 June 2021, blood test showed normal WBC count and improving platelet levels. The LNs were no longer palpable, and hepatosplenomegaly had resolved.
Last seen on 28 June 2023, the patient was well and remained on acalabrutinib treatment, with ongoing progression-free survival (PFS) of >2 years. His ECOG PS remained 0 and he continued to enjoy a good quality of life, with monthly travels.
Discussion
Guidelines of the National Comprehensive Cancer Network (NCCN) recommend A±O as one of the “preferred regimens” (category 1) for first-line treatment of CLL patients without del(17p) or TP53 mutation. Other “preferred regimens” include venetoclax + obinutuzumab and zanubrutinib, while chemoimmunotherapy (CIT) (eg, bendamustine + rituximab) is one of “other recommended regimens” for first-line treatment of this subset of patients.2
Patients who are stable at the time of CLL diagnosis do not require immediate treatment. Indications to initiate treatment include unintentional weight loss, significant cytopenia, progressive lymphocytosis (ie, lymphocyte doubling time of <6 months), or hepatosplenomegaly.3
The choice of A+O for our patient was based on the randomized, open-label, phase III ELEVATE-TN trial (n=535), where A±O significantly improved the primary endpoint of PFS vs CIT with obinutuzumab + chlorambucil (O+Clb) in patients with previously untreated CLL.4 In the interim analysis after a median follow-up of 28.3 months, median PFS was longer with A+O vs O+Clb (not reached [NR] vs 22.6 months; hazard ratio [HR], 0.1; 95 percent confidence interval [CI], 0.06–0.17; p<0.0001) and with acalabrutinib monotherapy vs O+Clb (NR vs 22.6 months; HR, 0.20; 95 percent CI; 0.13–0.3, p<0.0001).4 Notably, the superior PFS of acalabrutinib vs O+Clb was consistently maintained across high-risk genetic subgroups, such as patients with del(17p13.1), unmutated IGHV and complex karyotype. An even greater magnitude of benefit was observed with A+O in high-risk subgroups vs O+Clb.4
The PFS benefit of A±O vs O+Clb was maintained at the longer-term median follow-up of 58.2 months (median, NR vs 27.8 months) (A+O vs O+Clb: HR, 0.11; 95 percent CI, 0.07–0.16; p<0.0001) (acalabrutinib vs O+Clb: HR, 0.21; 95 percent CI, 0.15–0.30; p<0.0001), with consistent PFS findings across high-risk subgroups.5 (Figure)
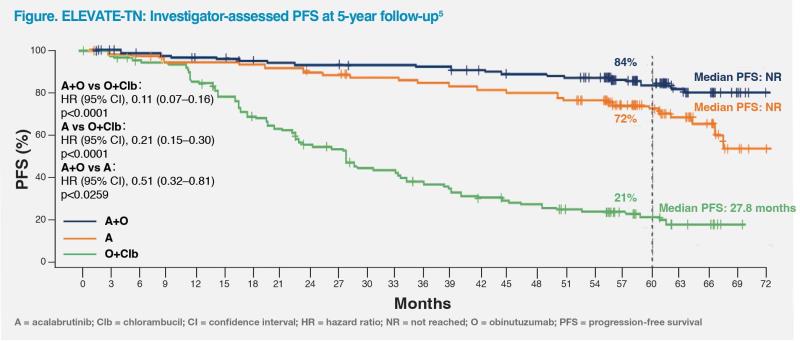
At 5 years, overall survival rate was significantly higher with A+O vs O+Clb (90 percent vs 82 percent; HR, 0.55; 95 percent CI, 0.30–0.99; p=0.0474).5 Sustained benefits of A+O vs O+Clb were also observed in overall response rate (96.1 percent vs 83.1 percent; p<0.0001) and rate of undetectable minimal residual disease (among patients who achieved complete response [including incomplete blood count recovery]) (42 percent vs 9 percent).5
Despite longer treatment exposure, acalabrutinib maintained an acceptable safety profile consistent with previous reports, with low rates of any-grade atrial fibrillation (AF) (A+O: 6.2 percent; acalabrutinib: 7.3 percent; O+Clb: 0.6 percent) and hypertension (9.6 percent, 8.9 percent and 3.6 percent, respectively) in the 5-year follow-up of ELEVATE-TN.5
In the head-to-head ELEVATE-RR trial, acalabrutinib was associated with less frequent any-grade AF (9.4 percent vs 16 percent; p=0.02), hypertension (9.4 percent vs 23.2 percent; p<0.001), and bleeding (38.0 percent vs 51.3 percent; p<0.05) vs ibrutinib.6 General precautions recommended for acalabrutinib include prophylaxis of tumour lysis syndrome, renal function tests, cautious use of anticoagulants (especially in the elderly), as well as bleeding and AF monitoring.
Initiation of A+O in our patient led to rapid shrinkage of LNs, which was accompanied by a transient increase in WBC count. This should not be taken as a sign of disease progression as it is a known effect of BTK inhibitors caused by mobilization of lymphocytes into the peripheral circulation.2
Our patient’s response to A+O was rapid and sustained, without significant adverse events, such as arrythmia or significant bleeding. While A±O improves PFS over CIT and offers a chemotherapy-free option for patients with treatment-naive CLL, acalabrutinib monotherapy may be considered in elderly patients 70–80 years of age in whom infection risk is a concern.