Although guideline-directed medical therapies (GDMTs) for heart failure (HF) with reduced ejection fraction (HFrEF) are effective in reducing risks of mortality and hospitalization for HF (HHF), the clinical burden of HF remains high. At a recent scientific symposium, Dr Yuya Matsue of Juntendo University Graduate School of Medicine in Japan discussed strategies of tackling worsening HF (WHF) to improve patient outcomes and presented a case study of vericiguat in HFrEF.
Worsening heart failure
Worsening HF can be defined as deterioration of HF signs and symptoms in patients with chronic HF despite continued therapy. [J Am Coll Cardiol 2023;81:413-424] It is recognized as a distinct phase in the trajectory of HF. “In a prospective observational Italian HF study [n=5,610], when compared with chronic HF patients and those with de novo HF, patients with WHF had higher rates of all-cause mortality [27.7 percent vs 5.9 percent and 19.2 percent; p<0.0001] and HHF [21.4 percent vs 8.8 percent and 8.2 percent; p<0.0001] at 1 year. In addition, a large cohort study [n=8,632] in Finland with a 12-year follow-up revealed that patients with more recent HHF were more likely to be rehospitalized, and the risk of HHF increased with each additional HHF event across a broad EF range,” Matsue said. [Circ Heart Fail 2013;6:473-481; ESC Heart Fail 2020;7:2406-2417]
Identifying patients at increased risk of worse prognosis
“A fundamental strategy in treating patients with HF is to estimate their overall risk profile and identify those who need more intensive monitoring and therapy,” said Matsue.
Apart from elevated N-terminal pro B-type natriuretic peptide (NT-proBNP), noncardiovascular (non-CV) comorbidities are associated with worse prognosis of HF. Data from a US-based HF registry of patients hospitalized with HF (n=207,984) indicated the risk of 30-day mortality and 30-day HF readmission after discharge increased with an increasing number of non-CV comorbidities (chronic obstructive pulmonary disease [COPD]/asthma, anaemia, diabetes mellitus, obesity [BMI ≥30 kg/m2] and renal impairment). [Circ Heart Fail 2018;11:e004646]
Physical signs of congestion are also strongly predictive of clinical outcomes in HF patients. In an analysis of the phase III trial comparing sacubitril/valsartan with enalapril in HFrEF (PARADIGM-HF), even after adjusting for baseline NT-proBNP measurements, Meta-Analysis Global Group in Chronic (MAGGIC) HF risk score, and New York Heart Association (NYHA) class, the number of signs of congestion (jugular venous distention, oedema, rales, and third heart sound) was positively associated with incidence rates for adverse CV outcomes. “Because of its clinical relevance and prognostic value, a comprehensive physical exam is particularly important for risk stratification and optimal outcomes especially when NT-proBNP testing is not available,” stressed Matsue. [Circulation 2019;140:1369-1379]
Underutilization of HF medications due to intolerance
Although all patients with HFrEF should receive the four pillars of HF therapy for their cumulative effects on risk reduction, some core components are underprescribed in clinical practice. An international prospective, observational, longitudinal survey of outpatients with chronic HFrEF (n=7,092) that investigated clinicians’ utilization of GDMT found that recommended HF medications were suboptimally prescribed or underdosed. Common reasons for non-prescription included hypotension associated with angiotensin-converting enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) and beta blockers (BBs), and hyperkalaemia or worsening renal function linked to ACEIs/ARBs and mineralocorticoid receptor antagonists (MRAs). [Eur J Heart Fail 2016;18:514-522]
In addition, most clinical trials have excluded patients with low blood pressure (BP) and estimated glomerular filtration rate (eGFR), thus limiting evidence of benefits and safety for this population in clinical settings. [Eur J Heart Fail 2021;23:872-881]
“In clinical practice, not all patients with HFrEF are taking all four pillars because of concerns regarding their effects on BP or serum potassium levels that require close monitoring, dose adjustments and gradual uptitration,” Matsue added.
The NO–sGC–cGMP pathway as a therapeutic target
HF is associated with impaired synthesis of nitric oxide (NO) and decreased activity of its receptor, soluble guanylate cyclase (sGC). Deficiency in cyclic guanosine monophosphate (cGMP) contributes to myocardial and vascular dysfunction, leading to progressive myocardial damage and inflammation. Vericiguat is a soluble sGC stimulator that restores cGMP deficiency by directly stimulating sGC, independently of and synergistically with NO, in the NO–sGC–cGMP signalling pathway, which may improve myocardial and vascular function. [Clin Transl Sci 2023;16:2458-2466]
Clinical evidence for sGC stimulator in worsening HF
The efficacy and safety of vericiguat was demonstrated in the phase III VICTORIA trial, which included 5,050 HFrEF patients who had left ventricular ejection fraction (LVEF) <45 percent and recent WHF (either HHF ≤6 months before randomization or intravenous diuretic therapy in the last ≤3 months without hospitalization). The VICTORIA population is similar to the US-based outpatient registry PINNACLE (Practice Innovation and Clinical Excellence) patients with WHF and LVEF <45 percent in terms of baseline characteristics and outcomes, and is representative of real-world HFrEF patients. [N Engl J Med 2020;382:1883-1893; J Card Fail 2021;27:1374-1381]
In this patient population at such high risk of clinical events, over a median of 10.8 months, vericiguat in addition to standard of care significantly reduced the primary composite endpoint of CV death or first HHF (hazard ratio [HR], 0.90; 95 percent confidence interval [CI], 0.82–0.98; p=0.02) vs placebo. The absolute event-rate reduction with vericiguat was 4.2 events per 100 patient-years, which translated to an annual number needed to treat of 24. Furthermore, reductions in the primary composite endpoint (HR, 0.85; 95 percent CI, 0.76–0.95) and its CV death (HR, 0.84; 95 percent CI, 0.71–0.99) and HHF (HR, 0.84; 95 percent CI, 0.75–0.95) components were observed in patients on vericiguat compared with subjects on placebo with baseline NT-proBNP levels ≤8,000 pg/mL. [N Engl J Med 2020;382:1883-1893; JACC Heart Fail 2020;8:931-939]
Minimal impact on BP and renal function
Vericiguat has a good safety profile. The impact of vericiguat on BP was minimal in VICTORIA. Likewise, no excessive BP reduction was observed with vericiguat in patients predisposed and potentially vulnerable to hypotension (ie, >75 years old, systolic BP 100–110 mm Hg or taking angiotensin receptor/neprilysin inhibitor [ARNI] at baseline). “Unlike ARNI, vericiguat is relatively easy to uptitrate in clinical practice because of its minimal effect on BP,” said Matsue. [N Engl J Med 2020;382:1883-1893; J Am Heart Assoc 2021;10:e021094]
Vericiguat also has little impact on renal function and electrolytes. The trajectories of serum creatinine (p=0.18) and eGFR (p=0.50) were similar between patients receiving vericiguat and placebo over the trial’s follow-up. Despite including patients with eGFR ≥15 mL/min/1.73 m2, the beneficial effects of vericiguat in VICTORIA were consistent across the full range of eGFR. Similarly, there were no clinically significant differences in changes in sodium or potassium levels between vericiguat- and placebo-treated patients. [Eur J Heart Fail 2021;23:1313-1321]
“Importantly, adherence to vericiguat was >80 percent in 94 percent of patients, and the median dose of vericiguat was 9.2 mg,” highlighted Matsue.
Optimal uptitration to achieve maximum benefit
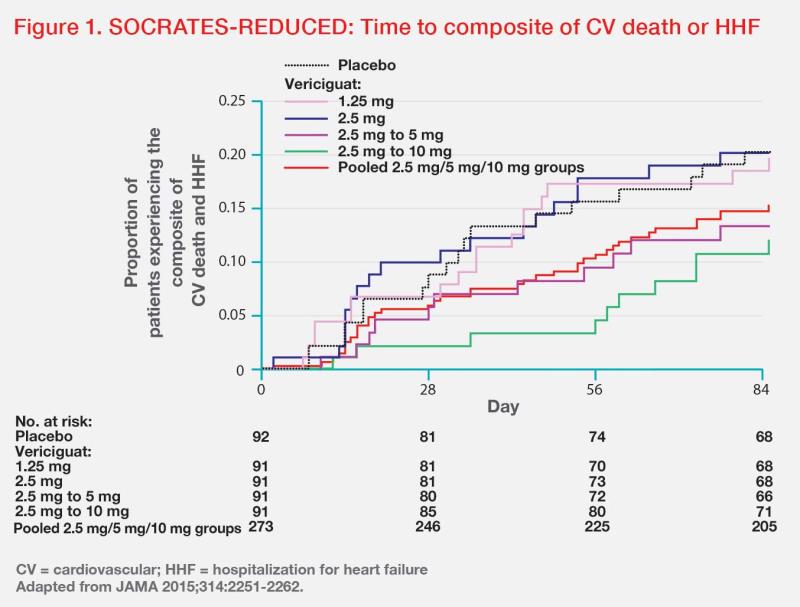
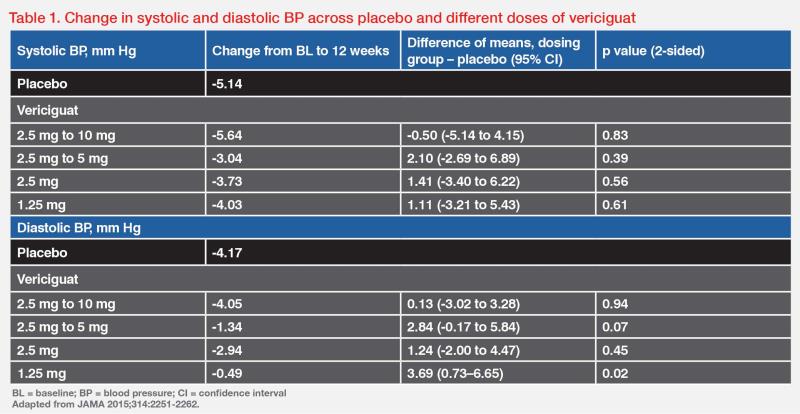
“Optimal uptitration of vericiguat to 10 mg QD is important to achieve its maximum benefit, as long as it is tolerated,” stressed Matsue. In the phase IIb randomized, dose-finding SOCRATES-REDUCED study comparing vericiguat from daily doses of 1.25 mg to 10 mg in patients with WHF and LVEF <45 percent (n=456), a dose-response relationship was suggested, with higher vericiguat doses associated with greater reductions in NT-proBNP level (p<0.02) and lower rates of the composite of CV death or HHF. (Figure 1) “More importantly, vericiguat did not cause any significant changes in mean systolic and diastolic BP from 1.25 mg to 10 mg QD compared with placebo,” noted Matsue. (Table 1) [JAMA 2015;314:2251-2262]
The evolving management of HF
Based on results of VICTORIA, the European Society of Cardiology (ESC) guidelines for diagnosis and treatment of acute and chronic HF recommend vericiguat to reduce risk of CV mortality or HHF in HFrEF patients who have WHF despite treatment with an ACEI (or ARNI), a BB and an MRA. [Eur Heart J 2021;42:3599-3726]
A clinical consensus statement by the Heart Failure Association of ESC also indicates that vericiguat administration should be advised, in addition to the four pillars of GDMT, in symptomatic patients with LVEF <45 percent after a WHF event. [Eur J Heart Fail 2023;25:776-791]