Kidney function improvement with an SGLT2 inhibitor in an elderly patient with CKD and T2DM
Specialist in Nephrology
The Chinese University of Hong Kong
Hong Kong

Presentation and management
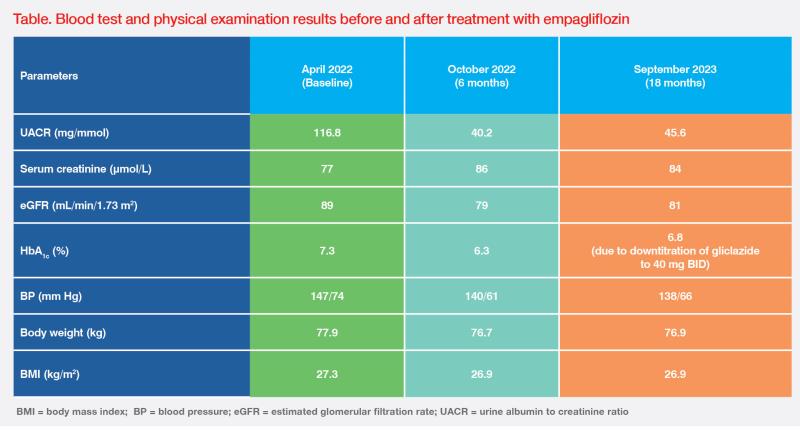
A 69-year-old male with a body mass index (BMI) of 27.3 kg/m2 presented in April 2022 with overt macroalbuminuria that had persisted for 1.5 years. He had long-standing history of type 2 diabetes mellitus (T2DM; HbA1c, 7.3 percent) and hypertension (systolic blood pressure [BP], 140–150 mm Hg) for >20 years, and was on antihypertensive treatment with losartan 100 mg QD, amlodipine 10 mg QD, metoprolol 100 mg BID, prazosin 2 mg BID, and methyldopa 250 mg BID as well as glucose-lowering medications with metformin 750 mg BID and gliclazide 80 mg BID.
Laboratory tests revealed severely elevated urine albumin-to-creatinine ratio (UACR; 117 mg/mmol) and mildly decreased estimated glomerular filtration rate (eGFR; 89 mL/min/1.73 m2), which were consistent with a diagnosis of chronic kidney disease (CKD; G2A3) at high risk of poor prognosis, according to the Kidney Disease: Improving Global Outcomes (KDIGO) classification.1
Even with a very high UACR, the patient was reluctant to change his treatment regimen because he was asymptomatic except for having long-standing frothy urine. After educating him on his risk of progression of CKD, he agreed to start empagliflozin 10 mg QD in April 2022.2 Following 6 months of empagliflozin treatment, his HbA1c, BP and BMI improved, together with substantial reduction in UACR (-66 percent). There was an initial dip in eGFR by approximately 10 percent (rise in serum creatinine), which then stabilized and was compatible with the known acute haemodyanimc effect of SGLT2 inhibitors.3 (Table)
Upon the last follow up in September 2023, the renal function and BP of this patient were stable. (Table) His HbA1c increased slightly after gliclazide was downtitrated to 40 mg BID due to concerns of weight gain and hypoglycaemia, but glycaemic control remained acceptable as per the American Diabetes Association (ADA) recommendation (HbA1c <7 percent).4 Most importantly, he tolerated empagliflozin very well, without any side effects, and will continue empagliflozin treatment with regular follow-up until requiring dialysis or transplantation, as per the ADA guidelines.4
Empagliflozin was selected for our patient based on guidelines of the European Association for the Study of Diabetes (EASD), which recommend initiating a sodium-glucose cotransporter 2 (SGLT2) inhibitor in CKD patients with eGFR ≥20 mL/min/1.73 m2 and UACR >3.0 mg/mmol to slow down CKD progression. Additionally, SGLT2 inhibitors are recommended in T2DM patients with high cardiovascular (CV) risk, comorbid heart failure (HF), or those who are overweight.7 Empagliflozin was chosen for our overweight T2DM patient with CKD (UACR, 116.8 mg/mmol), as it provides cardiorenal benefits and facilitates glycaemic goal achievement and weight management.
In the EMPA-REG OUTCOME trial that included T2DM patients at high CV risk (n=7,020), empagliflozin demonstrated a 39 percent relative risk reduction (RRR) vs placebo in incident or worsening nephropathy (hazard ratio [HR], 0.61; 95 percent confidence interval [CI], 0.53–0.70; p<0.001).8 It also significantly reduced annual eGFR loss vs placebo in patients with overt diabetic kidney disease (DKD; -1.60 mL/min/1.73 m2 vs -6.00 mL/min/1.73 m2), non-overt DKD (0.55 mL/min/1.73 m2 vs -0.74 mL/min/1.73 m2) and all others (-0.37 mL/min/1.73 m2 vs -1.48 mL/min/1.73 m2) (all p<0.001), suggesting efficacy in delaying renal function deterioration in T2DM patients irrespective of clinical DKD phenotype or presence of overt albuminuria.9
More recently, the EMPA-KIDNEY trial in CKD patients with or without diabetes (n=6,609) demonstrated a significant 28 percent RRR in the composite primary outcome of kidney disease progression (ESKD, sustained eGFR decrease to <10 mL/min/1.73m2, sustained eGFR decline of ≥40 percent, or renal death) or CV death with empagliflozin vs placebo (HR, 0.72; 95 percent CI 0.64–0.82; p<0.001). Renal benefits of empagliflozin were consistent among patients with or without diabetes at baseline and across a broad range of eGFR (down to ≥20 mL/min/1.73 m2).10 Notably, EMPA-KIDNEY encompassed a broader range of CKD patients than the DAPA-CKD trial (eGFR, 20–<90 mL/min/1.73 m2 vs 25–75 mL/min/1.73 m2; UACR, ≥200 mg/g or no limit [if eGFR <45 mL/min/1.73 m2] vs ≥200 mg/g), and may therefore be more reflective of CKD patients encountered in clinical practice.10,11
Empagliflozin’s renoprotective effects also extend to chronic HF patients across the spectrum of left ventricular ejection fraction (LVEF), as demonstrated in the EMPEROR-Preserved (LVEF >40 percent) and EMPEROR-Reduced (LVEF ≤40 percent) trials. In acute HF settings, use of empagliflozin was not shown to increase acute renal events in the EMPULSE trial.12-14
Empagliflozin is well tolerated. The most common adverse event (AE) is genital infection. In clinical trials, other AEs, including hypoglycaemia, volume depletion and diabetic ketoacidosis, occurred at similar rates vs placebo.2,8
Although SGLT2 inhibitors are recommended for T2DM patients with high CV risk and/or CKD, their use in these populations remains low. An interview-based study in Hong Kong found that primary care doctors are reluctant to prescribe SGLT2 inhibitors because they misperceive SGLT2 inhibitors merely as glucose-lowering agents and are not fully aware that their cardiorenal benefits are independent of glycaemic efficacy.15,16 Also, as seen in our patient with very high UACR who was otherwise asymptomatic except for having frothy urine, some patients are content with the status quo and unwilling to modify treatment to achieve targets even though they are at risk of developing ESKD.
The eGFR threshold for SGLT2 inhibitor use is ≥20 mL/min/1.73 m2 in patients with T2DM, CKD or HF.4,10,17 After empagliflozin treatment, our patient had improvement in kidney function along with improved glycaemic control and weight loss. His experience was consistent with results of published landmark trials, which together suggested the potential cardiorenal benefit of empagliflozin in patients with or without diabetes, across a broad range of kidney function.