Long-acting antiretroviral therapy in holistic management of an elderly patient with HIV
Specialist in Infectious Disease
Hong Kong

Presentation and investigations
A 70-year-old female garment factory worker presented with an incidental finding of HIV infection following a diagnosis of pulmonary tuberculosis (PTB). She was screened positive after her partner received a diagnosis of HIV/PTB coinfection.
The patient enjoyed good past health. She took pantoprazole 40 mg daily PRN for gastric reflux, and was not on any regular medication otherwise. She denied any history of commercial sex or recreational drug use and claimed to have a monogamous relationship with her partner. She had not been sexually active for >10 years, but she and her husband were reluctant to further dwell on the diagnosis of HIV or their sexual relationship.
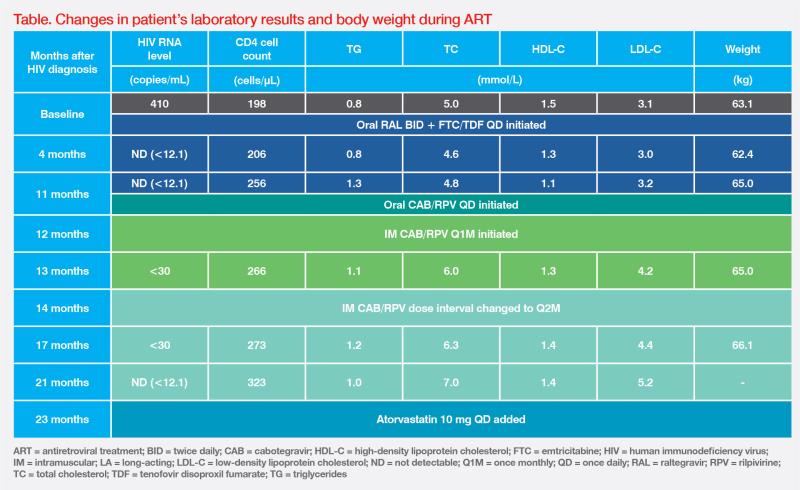
At presentation, the patient ̓s HIV viral load was 410 copies/mL and the CD4 cell count was 198 cells/μL. (Table) HIV genotypic resistance testing was not performed due to low level of viraemia. She screened positive for hepatitis B core antibody (anti-HBc) and hepatitis B surface antibody (anti-HBs), suggestive of previous hepatitis B virus (HBV) infection with seroconversion. Her HBV DNA level was not detectable.
Treatment and response
Standard tuberculosis (TB) therapy (ie, isoniazid, rifampicin, pyrazinamide, and ethambutol) was initiated 1 month before commencing multiple-tablet antiretroviral treatment (ART) with raltegravir (RAL) 400 mg BID (800 mg BID while on rifampicin) and emtricitabine (FTC) 200 mg plus tenofovir disoproxil fumarate (TDF) 300 mg QD. Viral suppression was achieved after 4 months of treatment. (Table)
Despite effective virologic control, the patient was dissatisfied with oral ART. It was a daily reminder of her HIV status and put a strain on her marriage. After TB treatment was completed, the option of long-acting (LA) injectable treatment with cabotegravir (CAB) and rilpivirine (RPV) was offered, and she decided to switch. She completed 4 weeks of CAB 30 mg + RPV 25 mg QD oral lead-in, then proceeded to intramuscular (IM) CAB 600 mg + RPV 900 mg Q1M for 2 months, followed by Q2M thereafter. During the oral lead-in period, pantoprazole was temporarily substituted by famotidine 40 mg BID to reduce drug-drug interactions with RPV.
After switching to LA CAB + RPV, the patient remained virally suppressed (ie, HIV RNA ≤50 copies/ mL), and her CD4 cell counts continued to rise to 323 cells/μL. (Table)
The patient’s body weight increased from 63 kg to 65 kg during ART. Such weight gain has been associated with a ‘return-to-health’ effect with successful HIV and TB treatment.1,2 After switching to LA CAB + RPV, her LDL cholesterol (LDL-C) level increased from 3.2 mmol/L to 5.2 mmol/L. This was likely due to discontinuation of TDF, which has a known lipid-lowering effect.3 Atorvastatin 10 mg QD was therefore added. (Table)
The patient’s mood improved with LA CAB + RPV treatment. During the last visit, she remained virally suppressed without evidence of treatment-emergent drug resistance. She also did not report any adverse events (AEs) or injection site reactions (ISRs).
Discussion
Holistic care for people living with HIV (PLHIV) should address their psychosocial challenges in addition to viral suppression. Its importance is reflected in the proposed additional treatment target of 90 percent of patients having a good quality of life (QoL) in the Joint United Nations Programme on HIV/AIDS ‘90-90-90’ targets.4
Our patient achieved effective viral suppression with oral ART, but her QoL worsened with the daily oral regimen, which was a constant reminder of her HIV status and could aggravate marital conflicts. Oral ART needs to be taken at the same time every day, which may be inconvenient and lead to unwanted disclosure of HIV status, stigma, and privacy concerns. Lifetime daily ART could lead to adherence anxiety and treatment fatigue. Consecutively missed doses are associated with HIV viral replication, resulting in drug resistance mutations.5-7 IM CAB + RPV with longer dosing intervals (Q1M or Q2M) can address many of these psychosocial challenges.6,8-10
The clinical decision to switch the patient to LA CAB + RPV was supported by two randomized trials: ATLAS-2M (Antiretroviral Therapy as Long-Acting Suppression every 2 Months) and SOLAR (Switch Onto Long-Acting Regimen).10-13
In ATLAS-2M, PLHIV treated with oral standard-of-care regimens were randomized 1:1 to receive LA CAB + RPV Q2M (n=522) or Q1M (n=523). The Q2M regimen was noninferior to the Q1M regimen for viral suppression at week 48. In the extension phase of ATLAS-2M, noninferior virologic efficacy of the Q2M vs Q1M regimen was maintained to week 152 (HIV-1 RNA ≥50 copies/mL, 2.7 percent vs 1.0 percent; adjusted treatment difference, 1.7 percent; 95 percent confidence interval [CI], 0.1–3.3).11,12
In SOLAR, PLHIV treated with oral bictegravir + emtricitabine + tenofovir alafenamide (BIC + FTC + TAF) were randomized to continue this regimen for 12 months (n=223) or switch to LA CAB + RPV Q2M (n=447). At months 11–12, LA CAB + RPV showed noninferiority to oral BIC + FTC + TAF for viral suppression (1 percent vs <1 percent; adjusted difference in proportion, 0.7; 95 percent CI, -0.7 to 2.0).13
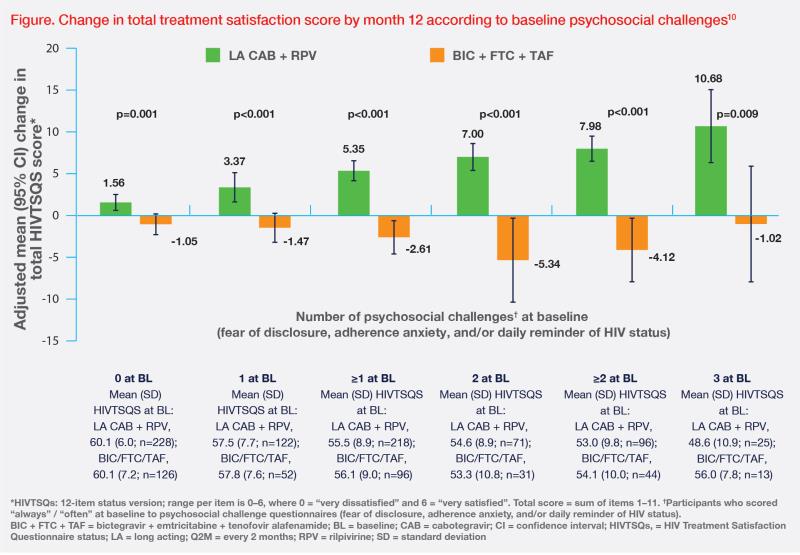
An analysis of patient-reported outcomes in SOLAR offers valuable insights into the psychosocial benefits of switching from a daily oral regimen to LA CAB + RPV. Despite a high satisfaction rate with oral therapy and being virally suppressed at baseline, 47 percent of patients reported ≥1 psychosocial challenge related to daily oral medications, including fear of disclosure, adherence anxiety, and daily reminder of HIV status, which was consistent with our patient’s experience.10
Switching to CAB + RPV Q2M led to statistically and clinically significant improvements in total HIV treatment satisfaction (HIVTSQs) at month 12 vs continuing oral BIC + FTC + TAF, which were driven mainly by treatment flexibility, satisfaction, and convenience.10 (Figure) In addition to longer dosing intervals, the flexibility of LA CAB + RPV is further enhanced by optional oral lead-in and a flexible ±7-day dosing window.8,9 At month 12, 90 percent of patients preferred LA CAB + RPV over continuing daily oral therapy (5 percent).10
Although not experienced by our patient, ISRs such as pain, induration, and nodule formation are commonly reported with LA CAB + RPV in clinical trial settings. These were generally mild, of grade 1 or 2, short in duration (median, 3 days), rarely caused treatment discontinuation, and became infrequent with time.11
Patients who may benefit from switching to LA ART include those facing psychosocial challenges or adherence issues, and those who find oral ART inconvenient due to work or travel commitments, such as frequent travellers and shift workers. As PLHIV require lifelong treatment, their preferences and lifestyles may change over time. The option of switching to LA ART can be discussed from time to time. Before switching, patients are required to be virologically suppressed, free from active HBV infection, and have no history of resistance to CAB or RPV.8,9,14
Confirmed virologic failure (CVF) was rarely reported in clinical trials (1.25 percent). Baseline risk factors for CVF included presence of ≥2 proviral RPV resistance–associated mutations, HIV-1 subtype A6/A1, and BMI ≥30 kg/m2. In patients with 0–1 baseline risk factor, the CVF rate was <0.5 percent. Additional consideration is warranted for those with ≥2 risk factors as they are at increased risk of CVF.15
Currently, barriers to broader use of LA CAB + RPV include lack of awareness among patients and lack of familiarity with the data among healthcare providers. The infrastructure of some clinics may also need to be adapted to the needs of patients on injectable regimens.
In summary, the efficacy of the Q1M and Q2M CAB + RPV regimens is comparable. Our case demonstrates the benefits of switching to LA ART, which is consistent with results of SOLAR and ATLAS-2M showing durable virologic suppression and good tolerability to LA CAB + RPV Q2M. Injectable LA CAB + RPV represents a paradigm shift in HIV management with improvements in patients’ QoL (the fourth 90), particularly for patients with low adherence or psychosocial burden when on daily oral ART.