
The focus of HIV care has shifted beyond viral suppression towards addressing the holistic needs of people living with HIV (PLHIV) as advances in HIV treatment have considerably extended the lifespan of these patients. At a scientific meeting organized by the Hong Kong Society for HIV Medicine, Dr Chia-Jui Yang of the Department of Internal Medicine, Far Eastern Memorial Hospital (FEMH), Taipei, Taiwan, discussed the importance of implementing patient-reported outcome measures (PROMs) to improve HIV care. Dr Beng Eu of the Prahran Market Clinic, Melbourne, Australia, highlighted a long-acting (LA) antiretroviral therapy (ART) regimen comprising 2-monthly injectable cabotegravir (Vocabria, GlaxoSmithKline) plus rilpivirine as a viable alternative to oral HIV treatment for potentially improving the overall quality of life (QoL) of PLHIV.
Achieving the fourth 90: Optimizing QoL in PLHIV
The availability of effective ART has markedly improved survival among PLHIV, whose life expectancy now approaches that of the general population. [EBioMedicine 2022;77:103896] However, PLHIV face numerous physical, emotional and psychosocial challenges in relation to daily oral ART. [AIDS Behav 2021;25:961-972] “Hence, the focus has now shifted from disease-centred care to patient-centred care,” said Yang. To achieve this, QoL has been proposed as the “fourth 90” to complement the Joint United Nations Programme on HIV/AIDS (UNAIDS) 90-90-90 testing and treatment targets. [BMC Med 2016;14:94]
“Patient-centred care incorporating patient-reported outcomes [PROs] can provide a more holistic view of treatment effectiveness and influence clinical decision-making,” said Yang. “PROs encompass various dimensions of care, including social wellbeing [eg, perception of HIV stigma], mental health, physical symptoms, health behaviours [eg, medication adherence, substance use], satisfaction with HIV care, and life circumstances [eg, housing, family/social support]. PROs are not directly observable and often not well addressed in routine clinical care due to time constraints. However, once identified, they are easily actionable.”
EACS 2023 guidelines on use of PROMs
The European AIDS Clinical Society (EACS) 2023 guidelines recommend utilization of PROMs annually in every individual to facilitate dialogue between healthcare providers and patients, improve both parties’ awareness of patients’ health conditions, introduce patient-centred care, and empower patients in this conversation. [EACS Guidelines version 12.0, October 2023]
“The guidelines also stress that PROM results should always be discussed during follow-up with a healthcare provider,” highlighted Yang.
Typical PROMs that assess health-related QoL (HRQoL) include EuroQoL 5-Dimension 5-Level (EQ-5D-5L) questionnaire, 36-Item Short Form Survey (SF- 36), and HIV-specific tools (eg, WHOQoL). “In our clinical practice, HIV-Symptom Index [HIV-SI] was the first questionnaire that we implemented as it was easier to translate from English to Chinese,” noted Yang. “We may then proceed to other tools, such as EQ-5D-5L, to explore other dimensions of challenges faced by PLHIV.”
How can PROs improve HIV care?
Studies have shown that PLHIV and their healthcare providers typically have different perceptions and priorities regarding HIV care. Patients are more likely to prioritize social context–based domains (eg, HIV stigma, social support), while providers tend to prioritize addressing behavioural domains, such as substance use. In addition, physicians often underestimate the symptom burden experienced by patients. [AIDS Behav 2020;24:1170-1180; Amador C, et al, EACS 2021 abstract PE5/24]
“PROs provide information that only PLHIV can deliver, such as perceptions, feelings and experiences,” noted Yang. “PROs can help identify barriers to or solutions for engagement and facilitate communication when creating a tailored care plan, which could empower PLHIV to take an active role in their care.” [Lancet HIV 2020;7:e59-e68; HIV Med 2019;20:542-554]
The use of PROs can improve HIV care provider documentation and prompt proactive actions. In an observational longitudinal cohort study of 1,083 PLHIV, provision of point-of-care PRO feedback to clinicians improved the accuracy of documentation of depression, poor medication adherence, and alcohol and substance use. In addition, providers were more likely to take further action for patients with comorbid depression or at-risk behaviours after receiving PRO feedback. [AIDS Behav 2017;21:3111-3121]
Digitalized PROMs experience in Taiwan
In 2023, the social communication app – LINE – was incorporated into the common online system of FEMH, Taiwan, to electronically collect PROs from PLHIV during routine follow-up visits. The HIV-SI questionnaire has been used as the PROM on this platform.
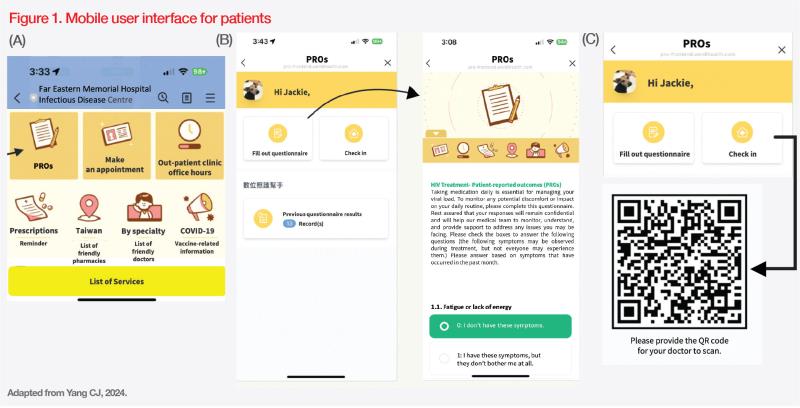
Initially, the HIV case manager explains the procedure to patients who are familiar with the use of smartphones and the LINE app. Before each consultation with an infectious disease specialist, patients can initiate the process by first selecting “PROs” from the menu on the mobile user interface. (Figure 1A) On the next screen, patients can choose “Fill out questionnaire”. (Figure 1B) Once the questionnaire is completed, answers are stored along with any previous evaluations done by the patient. These records can be accessed at any time through the patient’s mobile phone. Upon arriving at the outpatient clinic, the patient can select “check-in” on the screen, which will then display a unique QR code. (Figure 1C)
Before the patient arrives at the clinic, a tablet computer (with
the app installed) will be prepared in the consultation room for use by infectious disease specialists. During the follow-up visit, the physician can select “Patient check-in” on the app’s user interface and scan the patient’s QR code, which will reveal results of the questionnaire with PRO issues highlighted. (Figure 2)

Implementing digitalized PROMS offers many advantages. “Our patients complete the questionnaire with ease and doctors can access the records in the consultation room, which facilitates real-time communication with patients during each follow-up visit,” said Yang. “With properly stored past records, we can see the trend of each symptom over time. Psychosocial concerns or bothersome symptoms can be promptly addressed by offering specialist referrals and change of ART, which can improve patients’ QoL.”
“According to feedback received from our patients, it typically takes about 10–15 minutes to fill out the 20-item HIV-SI questionnaire the first time. However, it takes much less time thereafter,” he added.
New era of LA injectable HIV treatment
In phase III clinical trials, LA injectable cabotegravir (CAB) plus rilpivirine (RPV) treatment demonstrated noninferiority to daily oral ART for maintaining HIV suppression. Notably, LA CAB + RPV was also the preferred treatment among >90 percent of the trial’s participants. [Ramgopal MN, et al, CROI 2023, abstract 191; Lancet 2021;396:1994-2005; J Acquir Immune Defic Syndr 2020;85:498-506; Lutz T, et al, HIV Glasgow 2022, abstract P123; Czarnogorski M, et al, IAS 2021, abstract 881]
An international survey in 2,389 PLHIV uncovered several concerns associated with daily oral ART, including gastrointestinal side effects, comorbidities, fear of disclosure, stigma, anxiety regarding adherence to daily dosing, and the daily reminder of HIV status. “Among the survey’s respondents, 43.1 percent were interested in LA ART, which they wouldn’t have to take every day, while 54.7 percent expressed openness towards LA HIV Medication,” said Eu. [Popul Med 2020;2:23]
Participants from the CARISEL trial found that LA CAB + RPV can address key challenges associated with daily oral HIV Medication. After switching to LA treatment from standard oral therapy, a majority of patients reported less stigma (81 percent), lower adherence anxiety (71 percent), and not having to think about HIV status every day (67 percent). [Lutz T, et al, HIV Glasgow 2022, abstract P123]
Real-world studies of PLHIV who switched from oral ART to LA CAB + RPV also reported improvements in adherence and persistence, QoL, and preference for the LA ART over oral therapy. [Garris C, et al, IDWeek 2023, abstract 1024; Dandachi D, et al, IDWeek 2023, abstract 1567; Roucoux G, et al, EACS 2023, abstract eP.C3.029]
“Several LA and ultra-LA HIV Medications are currently under clinical development. However, CAB + RPV is likely to be the only LA ART available in the coming years,” pointed out Eu.
Who can benefit from LA CAB + RPV?
LA injectable CAB + RPV is suitable for adult HIV patients with appropriate HIV subtype, who are virologically suppressed with no significant baseline mutations and are able to commit to a 2-monthly schedule. [Vocabria Hong Kong Prescribing Information, February 2021]
The presence of baseline RPV resistance–associated mutations (RAMs), HIV-1 subtype A6/A1, and body mass index (BMI) ≥30 kg/m2 are associated with increased risk of confirmed virological failure (CVF) with LA CAB + RPV. [Orkin C, et al, HIV Glasgow 2022, abstract O44] “CVF is extremely rare in my clinical practice, and the risk of CVF in clinical trials was very low at 0.4 percent [in patients with 0–1 baseline factor],” added Eu. Only a combination of ≥2 baseline factors was associated with increased CVF risk. [AIDS 2021;35:1333-1342]
“It is important to discuss with our patients their physical and mental health and other life struggles they face to see if switching to LA CAB + RPV is appropriate,” noted Eu.
“Generally, individuals who contracted HIV within the past 15 years should be eligible for the injectable treatment as they are not likely to have developed multi-drug resistance, unlike those who were diagnosed much earlier [in the 1990s],” said Eu. “LA ART is particularly suitable for PLHIV who are not adherent to oral tablets. Patients who switch to LA ART can have better QoL without worrying about forgetting to take medication or accidental disclosure of their HIV status. They can also enjoy overseas travel without concern about custom restrictions on carrying their medications.”