Management of adults with SMA: Practical considerations and treatment experiences
Department of Neurology
Essen University Hospital
Essen, Germany, Dr. Shirley Pang
Division of Neurology
Queen Mary Hospital
Hong Kong

Spinal muscular atrophy (SMA) is an inherited disorder that causes progressive muscle degeneration and weakness. Although there is no cure for SMA, emerging disease-modifying treatments can potentially change disease trajectory and substantially improve patients’ outcomes. At a local symposium, Professor Tim Hagenacker of the Department of Neurology, Essen University Hospital in Essen, Germany, shared his clinical experience and expertise in managing adults with SMA, with a focus on early treatment with an oral drug, risdiplam (Evyrsdi®). Dr Shirley Pang of the Division of Neurology, Queen Mary Hospital, Hong Kong, reported preliminary data on treatment outcomes with risdiplam in Hong Kong.
SMA: Aetiology and phenotypes
SMA is an autosomal recessive disorder. Mutations in the survival motor neuron 1 (SMN1) gene inhibit the production of functional SMN protein in affected individuals, causing progressive muscle degeneration and weakness. A highly homologous SMN2 gene encodes a small amount of functional SMN protein and is present in all patients with varying numbers of copies. Higher numbers of SMN2 copies are associated with milder phenotypes. SMA is subdivided into three main subtypes (in decreasing severity: type I, II and III) based on age at symptom onset, motor milestones achieved, and clinical presentation. [J Neuromuscul Dis 2020;7:1-13]
Rationale for early SMA treatment
SMA is a lifelong progressive disease regardless of subtype or age at symptom onset. Natural history studies have documented progressive loss of muscle strength and motor function that often starts in childhood and continues into adulthood (average annual rate of decline of 1 point on the Medical Research Council [MRC] sum score and 0.5 points on the Hammersmith Functional Motor Scale Expanded [HFMSE] score). [Eur J Neurol 2018;25:512-518] Even patients with milder type III SMA (age, ≥20 years) experience a decline of almost 10 m/year on the 6-minute walk test (6MWT). For type III SMA patients, the interval between disease onset and loss of ambulation ranges from 15 years to 21 years. [PLoS One 2018;13:e0199657; Pediatrics 2004;114:e548-e553]
“Patients may not always notice their gradual decline in motor function between clinic visits. Therefore, it is important to inquire about any change in their condition from prior years,” noted Hagenacker.
“A majority of patients with SMA and their caregivers consider disease stabilization as a positive treatment result,” said Hagenacker. “Hence, one of our goals is to maintain residual motor function, which can promote social integration and enhance quality of life [QoL].” [Neuromuscul Disord 2017;27:428-438; BMC Neurol 2015;15:217]
Patients with SMA also face numerous challenges encompassing multiple organ systems. This requires a multidisciplinary management approach involving respiratory, gastrointestinal, orthopaedic and neurology specialists as well as dietitians and physical therapists, among others. [Neuromuscul Disord 2018;28:103-115]

Approved therapies
Risdiplam and nusinersen are the two treatment options currently available for adult SMA patients. Both agents increase the amount of functional SMN protein levels by modifying the splicing of the SMN2 gene. Risdiplam is an orally administered small molecule with systemic distribution. [ACS Med Chem Lett 2021;12:874-877] Nusinersen is an antisense oligonucleotide delivered directly into the central nervous system (CNS) via intrathecal injection. Administering nusinersen to a patient with severe scoliosis can be challenging and may require fluoroscopy for lumbar access, resulting in high lifelong cumulative radiation exposure. Lumbar puncture is also problematic in patients who have already undergone spinal fusion. [J Neuromuscul Dis 2020;7:1-13]
SUNFISH: 4-year efficacy data for risdiplam
Data from the 48-week open-label extension phase of the SUNFISH trial (part 2) demonstrated improvement or stabilization in motor function associated with risdiplam vs placebo. “Approximately 60–70 percent of patients remained stable based on the change from baseline in Motor Function Measure 32 [MFM-32] total score [≥0], whilst 30–40 percent maintained the marked improvement [≥3] attained in the first year,” pointed out Hagenacker. “Furthermore, there was sustained improvement in RULM score, which is sensitive to gross motor function in the upper extremities – an important measure for the non-ambulant wheelchair-dependent patient group.” [Oskoui M, et al, MDA 2023]
“In line with the above results, patients and caregivers also reported stabilization or continuous improvements in SMA Independence Scale–Upper Limb Module [SMAIS-ULM] total score. This is an important patient-reported outcome [PRO] measure that reflects patients’ ability to perform daily tasks, such as bathing and brushing teeth,” highlighted Hagenacker. [Oskoui M, et al, MDA 2023]
Treatment with risdiplam: Hong Kong experience Drug reimbursement and eligibility
In December 2020, risdiplam became available in Hong Kong through the Compassionate Use Programme (CUP) for type I and type II SMA patients. In late 2021, risdiplam received regulatory approval, and by the end of 2022, it was adopted into the Hospital Authority Drug Formulary with safety net for eligible patients in Hong Kong. [Pang YY, 2023; www.ha.org.hk/hadf/en-us/Updated-HA-Drug-Formulary/Drug-Formulary.html]
Notably, risdiplam is the only available treatment for adult SMA patients (eligibility: types I, II and III and age ≤25 years) under the current reimbursement policy. Patients on permanent ventilation in the absence of acute reversible illness or tracheostomy requirement at baseline are not eligible for reimbursement. Furthermore, patients with evidence of disease progression despite treatment who have no potentially reversible concurrent illness will be withdrawn from treatment upon yearly review.
Risdiplam in adult SMA patients
By 2022, 20 treatment-naive adult SMA patients (median age, 30 years; male, n=11; female, n=9; type II, n=19; type I, n=1) in Hong Kong had been enrolled into the CUP and treated with risdiplam for a mean duration of 13 months. At the beginning of treatment, the mean (range) values for MFM-32 total score, HFMSE total score and RULM score were 18.68 (1–44), 1.26 (0–5) and 7.28 (0–16), respectively. “These very low scores reflect how weak and severely affected our adult SMA patients were at the start of treatment,” noted Pang. “Particularly, the single-digit values for the HFMSE score highlight that this scale is not sensitive for this group of patients.” [Pang YY, 2023]
At the end of the treatment period, 18 patients had completed two assessments. Most patients stayed the same or improved on the HFMSE (mean change, 0.28 [-2 to 2]) and MFM-32 (mean change, 2 [-2 to 10]) scales. The RULM score also showed a similar trend. (Figure)
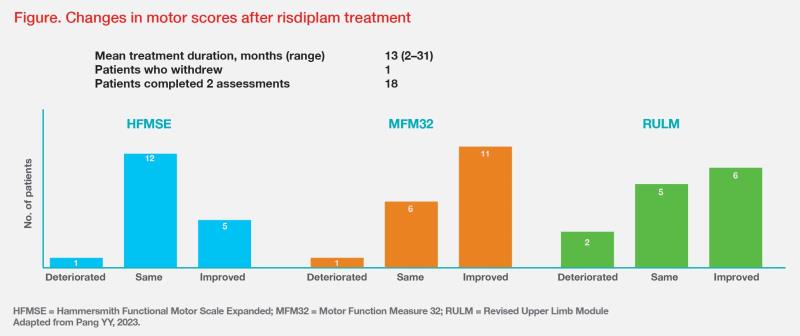
“Among 11 patients who showed improvement in the MFM-32 scale, five [25 percent of all patients] attained a clinically meaningful improvement of ≥3 points,” highlighted Pang. At 1-year follow-up, patients reported improvements in several PRO measures. (Table) “Even small improvements in these outcome measures are invaluable as they enable patients to become more self-reliant,” said Pang.
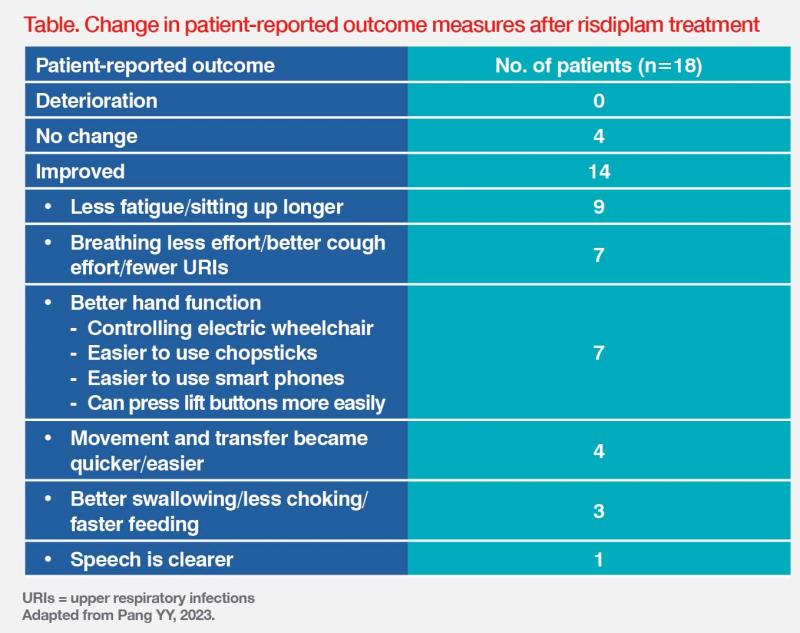
“Usual motor function assessments do not capture additional symptoms or impacts, such as endurance/fatigue, pain, speech [volume, pitch and clarity], and sleep. Therefore, we need more sensitive scales that can capture improvements in measures that are more relevant and meaningful for patients with SMA.” [Pang YY, 2023; BMC Neurol 2021;21:143]
Upon treatment initiation, some patients reported diarrhoea, which typically resolved within a few weeks. No patients discontinued treatment due to adverse events.
Summary
SMA is a lifelong progressive disease. Therefore, treatment should be initiated as early as possible to slow the rate of disease progression and to stabilize and/or improve motor function. In severely disabled patients, even small improvements can have a major impact on QoL. Risdiplamtreated SMA patients consistently show improvement or stabilization in both objective motor function assessments and subjective PROs.