
Positive results of (neo)adjuvant immunotherapy (IO) trials have enabled earlier use of multimodality treatment in non-small-cell lung cancer (NSCLC), which was previously reserved for more advanced disease. At a recent local symposium, oncology and surgery experts discussed latest treatment advances (eg, the IMpower010 trial of adjuvant atezolizumab), redefined unresectability in early-stage disease, highlighted expanded opportunities of cure, and explored hidden costs of neoadjuvant IO.
Entering the multimodality treatment era
“Previously, early-stage NSCLC was treated by surgeons, whereas advanced disease was oncologists’ territory. There was minimal overlap between the two specialties,” said Dr Alan Sihoe, Consultant in Cardio-Thoracic Surgery, Chinese University of Hong Kong (CUHK) Medical Centre. “We have now entered a new era where multimodality treatment is extended to earlier disease stages,” said Dr Johnny Kin-Sang Lau, Specialist in Clinical Oncology in private practice in Hong Kong. “Adding IO to standard treatment has improved 5-year overall survival [OS] rates of patients with stage II–III NSCLC to a median of 84.8 percent for resectable PD-L1–high disease.” [Brunelli A, et al, ESTS 2023, abstract O-026]
IMpower010: First positive adjuvant IO trial in NSCLC
“The IMpower010 trial is the first phase III adjuvant IO trial in early-stage NSCLC,” noted Lau. [J Hematol Oncol 2023;16:40] After up to four cycles of cisplatin-based chemotherapy, 1,005 patients with completely resected stage IB (tumour size ≥4cm)–IIIA NSCLC were randomized 1:1 to receive adjuvant intravenous (IV) atezolizumab 1,200 mg Q3W for 16 cycles or best supportive care (BSC). [Lancet 2021;398:1344-1357]
After a median follow-up of 32.2 months, adjuvant atezolizumab significantly reduced the risk of recurrence or death by 57 percent vs BSC (hazard ratio [HR], 0.43; 95 percent confidence interval [CI], 0.27–0.68) in stage II–IIIA patients with high PD-L1 expression (tumour cell [TC] expression, ≥50 percent).
Notably, the first interim overall survival (OS) analysis (data cut-off, 18 April 2022) showed an OS trend favouring adjuvant atezolizumab vs BSC (5-year OS rate, 84.8 percent vs 67.5 percent; HR, 0.42; 95 percent CI, 0.23–0.78; p=0.005) in the high PD-L1 expression population without EGFR or ALK alterations. The OS curves separated early and remained separated throughout the study. (Figure) [Ann Oncol 2023;34:907-919; Brunelli A, et al, ESTS 2023, abstract O-026]
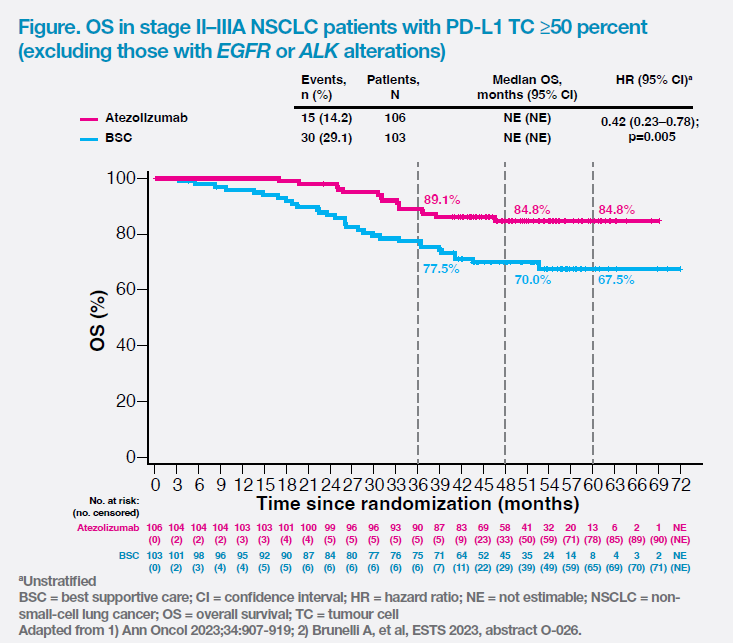
KEYNOTE-091 was another trial that assessed adjuvant IO in a similar patient population. “However, disease-free survival [DFS] data of pembrolizumab vs placebo [HR, 0.82; 95 percent CI, 0.57–1.18; p=0.14] in the high PD-L1 expression subgroup warrants further investigation,” noted Lau. [Lancet Oncol 2022;23:1274-1286]
What do surgeons bring to the table?
Redefine unresectable disease
The PACIFIC study, which showed a 5- year OS rate of 42.9 percent with durvalumab in patients with stage III unresectable NSCLC, raises questions on the role of surgery in stage III NSCLC. [J Clin Oncol 2022;40:1301-1311] “If a stage III patient has high PD-L1 expression and potentially resectable disease, do we still offer no surgery or surgery plus adjuvant atezolizumab, which could potentially improve OS significantly?” asked Lau.
“In the last decade, remarkable progress was seen in minimally invasive surgery [MIS], including uniportal video-assisted thoracoscopic surgery [VATS] for minimizing surgical access trauma, and sublobar resection for preserving more lung tissue with comparable oncological outcomes vs traditional lobectomy,” highlighted Sihoe. “Compared with thoracotomy, VATS was associated with a lower level of postoperative circulating tumour DNA, which was strongly correlated with pathologic response and reduced radiographic tumour size in the IMpower010 trial, indicating that type of surgery may directly influence efficacy of subsequent adjuvant therapy.” Enhanced recovery after surgery is another advancement that reduces length of hospital stay and postoperative complications. [Ann Cardiothorac Surg 2023;12:82-90; World J Surg 2023;47:1320-1322; BMJ Open Respir Res 2021;8:e000917; Ann Onco 2021;32:S1373-S1391; Innovations (Phila) 2016;11:179-186]
“With the latest advancements, the definition of ‘unresectable disease’ is very different from what it was 5–10 years ago,” emphasized Sihoe. “There are no longer absolute contraindications for curative surgery, even for the elderly and frail patients with multiple comorbidities.”
Expand opportunities for cure
Emerging studies revealed that surgery as part of multimodality therapy may be effective in advanced NSCLC. In patients with oligometastatic disease, local consolidative therapy with surgery or radiotherapy improved OS (median, 41.2 months vs 17.0 months; p=0.017) vs maintenance therapy or observation. Surgery + tyrosine kinase inhibitor ± chemotherapy resulted in a 3-year OS rate of 76.3 percent in stage IV NSCLC. In unresectable stage III patients who failed or responded insufficiently to initial chemoradiotherapy, salvage surgery achieved 5-year OS rates of 20–78 percent. [J Clin Oncol 2019;37:1558-1565; J Thorac Dis 2019;11:5463-5473; Transl Lung Cancer Res 2021;10:555–562] “In the era of multimodality therapy, we should be looking for better opportunities to treat patients whom we previously would have given up on,” said Sihoe.
Reveal hidden costs of neoadjuvant IO
“Results of neoadjuvant IO trials paint a rosy picture, but there are hidden costs,” pointed out Sihoe.
Of note, the surgical attrition rate can be as high as 46 percent in neoadjuvant IO trials. [J Thorac Cardiovasc Surg 2020;160:1376-1382] “This is in the context of clinical trials where patients are encouraged to adhere to treatment. The surgical attrition rate is anticipated to be higher in the real world,” said Sihoe.“It is worrisome that a high proportion of patients start induction IO, but never get curative surgery.”
In CheckMate 816, there were high rates of thoracotomy (59.1 percent) and conversion from MIS to thoracotomy (11.4 percent) in patients receiving neoadjuvant IO. “Notably, 16.8 percent of patients treated with IO plus chemotherapy underwent pneumonectomy – a procedure that should effectively be banned,” highlighted Sihoe. “The data are terrifying for modern-day thoracic surgeons as MIS should be the standard treatment for early-stage NSCLC, but the MIS rate was surprisingly low [29.5 percent] in those receiving neoadjuvant IO.” [N Engl J Med 2022;386:1973-1985]
“The inflammatory response to neoadjuvant IO resulted in fibrosis and adhesions, leading to dissection difficulties and higher rates of thoracotomy [eg, pneumonectomy], which are associated with increased postoperative pain, longer hospital stays, and a higher risk of complications vs MIS,” explained Sihoe. [Transl Lung Cancer Res 2021;10:3713-3736; Cancers (Basel) 2023; 15:2630]
“Neoadjuvant IO trials in NSCLC have shown promising data in terms of major pathological response. While the correlation between survival gains and pathological response to neoadjuvant IO were eagerly awaited, future neoadjuvant IO trials may need better definition of resectability and subject inclusion criteria to address the relatively high rates of thoracotomy and surgical attrition,” commented Lau. In contrast, IMpower010 provides strong evidence (eg, OS and DFS) for the use of adjuvant atezolizumab in early-stage NSCLC patients with high PD-L1 expression without surgical delay.