Use of the interleukin-31 receptor A monoclonal antibody nemolizumab significantly reduced the severity of pruritus in patients with moderate-to-severe prurigo nodularis, a phase II trial has shown.
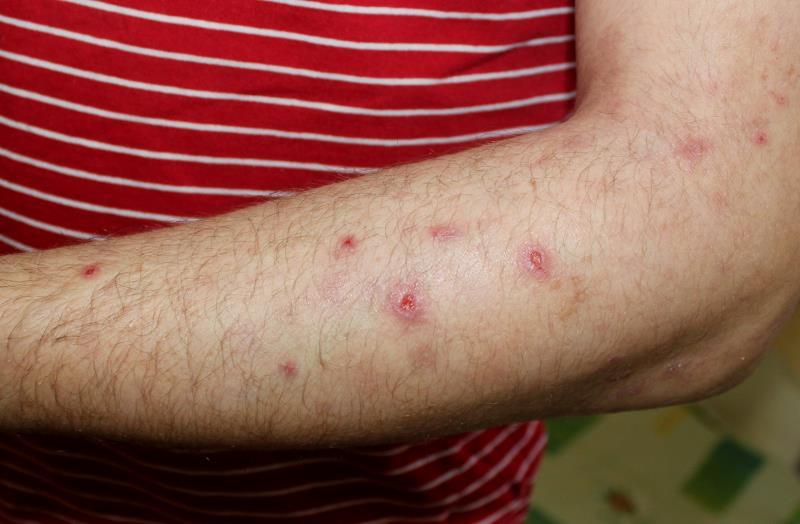
Prurigo nodularis is a treatment-refractory, potentially debilitating skin condition characterized by pruritic, hyperkeratotic lesions with a generalized pattern of distribution typically appearing along the arms, legs, or torso. [J Eur Acad Dermatol Venereol 2018;32:1059-1065; J Am Acad Dermatol 2018;79:714-719.e3]
“[The] treatment of prurigo nodularis mainly targets the symptom of pruritus [as] the intensity of pruritus in prurigo nodularis is considered … the highest among several types of chronic pruritic skin disease,” noted the researchers. [Acta Derm Venereol 2018;98:173-179; Dermatol Clin 2018;36:189-197; J Am Acad Dermatol 2018;79:457-463.e5; Eur J Pain 2016;20:37-40] The appearance and intense itchiness may trigger mental health issues (eg, anxiety, depression, suicidal ideation) and consequently impair quality of life (QoL) [J Eur Acad Dermatol Venereol 2019;33:157-162; https://rarediseases.info.nih.gov/diseases/7480/prurigo-nodularis, accessed March 18, 2020]
The team randomized 70 patients 1:1 to receive three subcutaneous injections of nemolizumab 0.5 mg/kg of body weight or placebo. The study drug was administered at baseline and at weeks 4 and 8. Intervention period ran for 12 weeks; follow up ensued for another 6 weeks. [N Engl J Med 2020;382:706-716]
At week 4, peak pruritus score on the numerical rating scale significantly dropped from baseline with nemolizumab vs placebo (-4.5 vs -1.7 points), translating to a greater percent change in the mean peak score (-53.0 percent vs -20.2 percent; p<0.001).
By week 12, nine nemolizumab recipients reported that ≥75 percent of their lesions have healed, as opposed to only two placebo recipients. Moreover, two nemolizumab recipients reported complete resolution of their lesions. Taking all healed lesions into context, lesion count was greatly reduced with nemolizumab vs placebo (least-squares mean [LSM], -12.6 vs -6.1 lesions), which persisted until week 18 (LSM, -13.3 vs -7.5 lesions).
“The patients … had had the disease for longer than 6 months … [These findings suggest that] more than one-third of [nemolizumab recipients] were clear or almost clear of lesions within a period of 3 months,” said the researchers.
Improvements in the numerical rating scale score for sleep quality was already evident by week 1 in favour of nemolizumab (LSM, -23.4 vs -5.1). By week 4, the percent change for this parameter was numerically greater with nemolizumab vs placebo (-56.4 percent vs -26.6 percent).
However, while this represents a QoL improvement, the researchers cautioned against making inferences based on the sleep quality data, as well as the lesion count, given the lack of multiplicity adjustments.
Despite similar overall AEs rates between the nemolizumab and placebo arms (68 percent vs 67 percent), nemolizumab use led to more gastrointestinal events such as abdominal pain and diarrhoea (21 percent vs 14 percent) and musculoskeletal symptoms (18 percent vs 14 percent).
Overall, the study supports the role of the pruritogen interleukin-31 in the pathophysiology of prurigo nodularis, noted the researchers. [Acta Derm Venereol 2019;99:579-586] “Interleukin-31, when bound to its receptor complex, has been shown to be a mediator of pruritus, especially in atopic dermatitis and prurigo nodularis. [Therefore,] inhibition of interleukin-31 signalling may be a target for treatment in these pruritic skin diseases.” [J Allergy Clin Immunol 2018;141:858-866; Nat Biomed Eng 2019;3:114-125; Br J Dermatol 2018;179:669-678]
The researchers called for larger and longer trials to validate the durability and safety of nemolizumab in this setting.