Case 1: Young patient with FH
History, presentation and treatment
A 27-year-old male was admitted to hospital on 29 April 2023 due to sudden-onset severe chest pain and shortness of breath on his way to work. He had no notable past medical history. ECG showed ST elevation over inferior leads. His high-sensitivity cardiac troponin T was extremely elevated at 1,902 ng/L. He was diagnosed with acute myocardial infarction (MI).
Urgent coronary angiography showed total occlusion of the right coronary artery. Primary percutaneous coronary intervention (PCI) with stenting to the right coronary artery was performed. The procedure was uneventful, and the patient was transferred to the medical ward for further management.
Blood tests on 29 April 2023 showed total cholesterol (TC) of 9.6 mmol/L, HDL-cholesterol (HDL-C) of 1.3 mmol/L, LDL-cholesterol (LDL-C) of 7.9 mmol/L, and TC/HDL-C ratio of 7.4. The patient’s BMI was normal, as was his lipoprotein(a) level. He was diagnosed with familial hypercholesterolaemia (FH) on the basis of these findings, although no genetic testing was carried out at the time in view of high cost.
Upon discharge on 4 May 2023, the patient was prescribed aspirin 80 mg QD, ticagrelor 90 mg BID, pantoprazole 40 mg QD, atorvastatin 40 mg QD, and ezetimibe 10 mg QD. Follow-up blood test on 4 June 2023 showed TC of 3.9 mmol/L, HDL-C of 1.3 mmol/L, LDL-C of 2.5 mmol/L, and TC/HDL-C ratio of 3.0.
Although the patient’s LDL-C level decreased considerably following treatment initiation, it still exceeded the target of <1.4 mmol/L plus ≥50 percent reduction from baseline recommended for very-high-risk patients in the latest 2022 consensus of the American College of Cardiology.1 Therefore, the patient commenced treatment with a proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitor, evolocumab (subcutaneous [SC] 420 mg Q4W), on 4 June 2023. The next test on 8 July 2023 showed TC of 2.2 mmol/L, HDL-C of 1.6 mmol/L, LDL-C of 0.6 mmol/L, and TC/HDL-C ratio of 1.4.
The patient tolerated his treatment well and reported no adverse events (AEs). Last measured on 4 September 2023, his LDL-C had further improved to 0.4 mmol/L.
Discussion
Elevated LDL-C level is associated with an increased risk of adverse ischaemic outcomes in the early post–acute coronary syndrome period.2 However, LDL-C levels recommended by international guidelines for secondary prevention in high-risk patients are not typically achieved within 4 weeks of initiating high-dose statins.2 Although our patient’s LDL-C level decreased from 7.9 mmol/L to 2.5 mmol/L after 1 month of treatment with atorvastatin 40 mg QD and ezetimibe 10 mg QD, it was still above the recommended target of <1.4 mmol/L plus ≥50 percent LDL-C reduction from baseline for very-high-risk patients.1,3 On the other hand, he was able to achieve the recommended LDL-C target after a single dose of evolocumab.
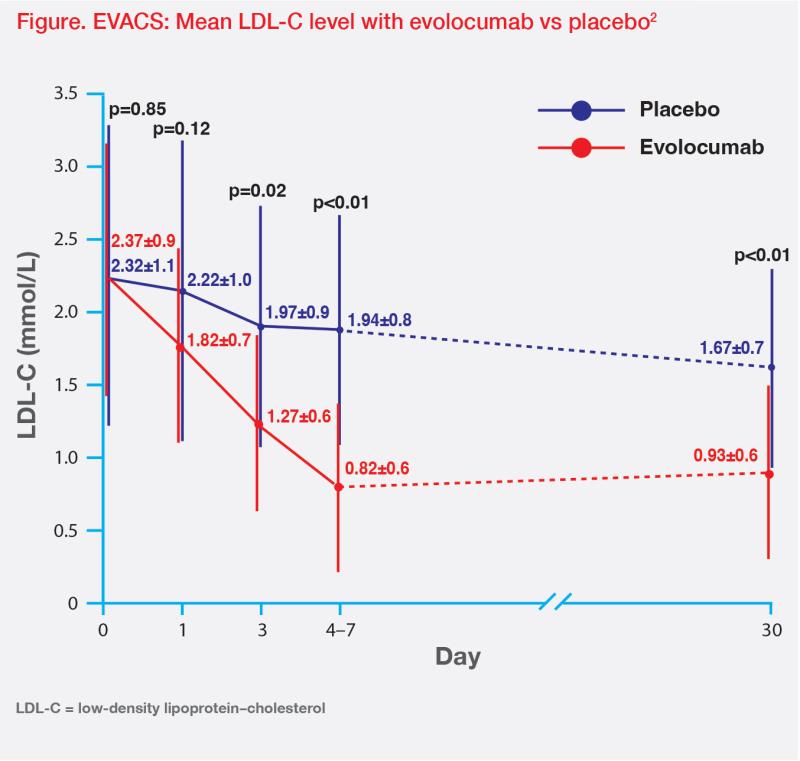
In the EVACS trial, 272 patients with MI were randomized 1:1 to receive a single dose of evolocumab SC 420 mg or matching placebo within 24 hours of presentation. All patients received high-intensity statins unless contraindicated.2 LDL-C decreased from baseline by day 1 in the evolocumab group (70.4 mg/dL or 0.79 mmol/L; p<0.01 vs baseline) and was significantly lower than that in the placebo group by day 3 (p=0.02 vs placebo). The between-group difference persisted throughout hospitalization and at day 30 follow-up (p<0.01).2 (Figure)
Since patients with FH require medication to control LDL-C levels for most of their lifetime, long-term efficacy data are important.
Evolocumab’s long-term ability to sustain LDL-C lowering was confirmed in the open-label, single-arm TAUSSIG trial, where 300 patients with homozygous FH (HoFH) or severe heterozygous FH (HeFH) received evolocumab for a median of 4.1 years.4 Mean reduction in LDL-C from baseline to week 12 was 21.2 percent and 54.9 percent in patients with HoFH and HeFH, respectively, with LDL-C persistently reduced through week 216.4
In TAUSSIG, evolocumab’s most common treatment-emergent AEs were nasopharyngitis (17.7 percent), influenza (12.7 percent), upper respiratory tract infection (11.7 percent), headache (11.3 percent), myalgia (10.0 percent), and diarrhoea (10.0 percent).4 Like present case, most patients in our practice do not report any AEs while on evolocumab.
Case 2: Elderly patient with statin intolerance
History, presentation and treatment
This 78-year-old male patient with a strong family history of hypertension (HTN) was first diagnosed with HTN at 52 years of age in 1997. His blood pressure (BP) was well controlled with a fixed-dose combination (FDC) of atenolol/chlortalidone 50 mg/12.5 mg QD until September 2020, when he presented to our clinic with systolic BP of 110–150 mm Hg and diastolic BP of 65–70 mm Hg, according to home measurements. Amlodipine 5 mg QD was added to the FDC medication.
Blood tests at the time revealed TC of 5.3 mmol/L, HDL-C of 1.2 mmol/L and LDL-C of 3.5 mmol/L, indicating borderline hyperlipidaemia. His BMI was normal.
CT coronary angiography on 22 July 2021 yielded a calcium score of 452, with mixed plaque in the mid-left anterior descending (LAD) coronary artery, calcified plaque at origin of posterolateral branch of the left circumflex coronary artery causing 50–70 percent stenosis in posterior/distal-LAD and mild stenosis in the proximal/mid-right coronary artery. The patient was advised to undergo PCI, but he refused.
He was put on aspirin 80 mg daily and rosuvastatin 10 mg QD on 27 July 2021. A check-up on 9 November 2021 showed good LDL-C response and normal serum creatine phosphokinase (CPK) level. His BP decreased to 125–135/50–60 mm Hg.
At the next follow-up on 5 May 2022, the patient’s LDL-C and CPK levels remained normal. However, in June 2022, he complained of neck and shoulder pain, while reporting being very active and lifting weights almost daily. Although his CPK levels remained in the normal range since commencing rosuvastatin, the patient believed his muscle pain was related to statin treatment and discontinued rosuvastatin in July 2021. He agreed to start treatment with evolocumab 140 mg SC Q2W in mid-August 2022 to control his hyperlipidaemia.
A test on 1 September 2022 showed markedly reduced LDL-C level of 0.7 mmol/L and CPK level of 523 IU/L. Another test on 23 November 2022 showed that most lipid values remained broadly stable, while CPK level had returned to normal range. At the same time, the patient reported taking a break from weight-lifting due to ongoing muscle pain.
In December 2022, the patient requested to extend evolocumab’s dosing interval to Q4W. Subsequent blood test on 8 March 2023 showed a mild increase in LDL-C to 0.9 mmol/L, but on 26 June 2023, it rose to 1.7 mmol/L. At this time, he had resumed physical exercise, albeit with lower weights.
Feeling that his muscle pain had not resolved completely 11 months after cessation of rosuvastatin, the patient intended to switch back to statin treatment. However, after a discussion on preventing further elevation of his LDL-C levels, the patient instead agreed to shorten evolocumab’s dosing interval. His latest LDL-C measurement taken in December 2023 was 0.9 mmol/L and CPK was 210 IU/L.
Discussion
Statins are the cornerstone of hyperlipidaemia management due to their established ability to reduce morbidity and mortality associated with atherosclerotic vascular disease.5 However, statin intolerance, which is primarily associated with “flu-like” muscle aches and weakness, is reported in 7–29 percent of patients, including our patient.6 PCSK9 inhibitors offer a feasible alternative, as they have been shown to substantially lower LDL-C levels and are typically well tolerated, with a low incidence of muscle-related AEs.7
GAUSS-3 was a randomized clinical trial in patients with elevated LDL-C levels who were unable to tolerate an effective statin dose because of muscle-related AEs. A total of 218 patients who experienced muscle-related AEs while taking atorvastatin but not placebo were randomized to receive oral ezetimibe plus SC placebo (n=73) or SC evolocumab plus oral placebo (n=145).8 The first coprimary endpoint of mean LDL-C level for the mean of weeks 22 and 24 was 2.1 mmol/L (least-squares mean [LSM] percent change from baseline, -16.7 percent) for ezetimibe and 1.2 mmol/L (LSM percent change, -54.5 percent) for evolocumab (p<0.001).8
Any muscle-related AEs occurred in 28.8 percent and 20.7 percent of patients on ezetimibe and evolocumab, respectively. Both ezetimibe and evolocumab were well tolerated during the trial, with 6.8 percent of ezetimibe- and 0.7 percent of evolocumab-treated patients discontinuing active treatment because of muscle-related AEs. Increases in creatine kinase levels occurred in 1.4 percent and 2.8 percent of patients on ezetimibe and evolocumab, respectively.8 However, it must be noted that many patients’ creatine levels remain normal even as they experience muscle symptoms, as did our patient’s.6
References:
- J Am Coll Cardiol 2022;80:1366-1418.
- Circulation 2020;142:419-421.
- Eur Heart J 2020;41:111-188.
- J Am Coll Cardiol 2020;75:565-574.
- Arch Toxicol 2023;97:1529-1545.
- Eur Heart J 2015;36:1012-1022.
- Fed Pract 2023;40:62-67.
- JAMA 2016;315:1580-1590.
The above content is for medical education purpose supported by Amgen.
HKG-145-0224-80002 Feb 2024