Rapid response with a CDK4/6 inhibitor in an elderly patient with HR-positive, HER2-negative advanced breast cancer and comorbidities
Department of Clinical Oncology
School of Clinical Medicine
University of Hong Kong

History, presentation and treatments
The patient was a 70-year-old female who presented with a large, fungating left breast mass in September 2022. Although first symptoms were noticed approximately a year earlier, the patient did not seek consultation sooner for fear of cancer diagnosis. Upon examination, the mass occupied the entire left breast. The patient also suffered from shortness of breath and left breast pain. These symptoms worsened, resulting in hospitalization.
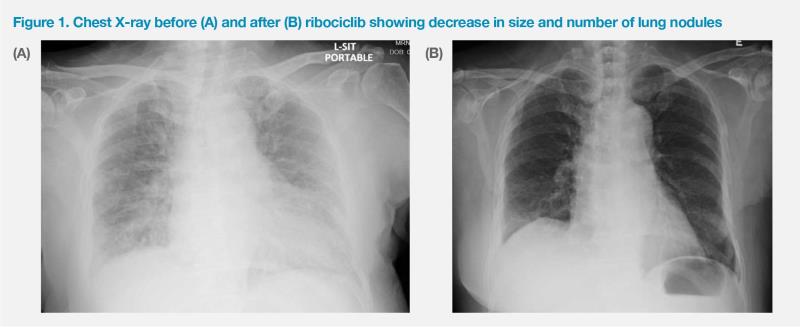
At the time of hospital admission, the patient’s condition was poor, with Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 2. She required supplemental oxygen of 3 L/min via nasal cannula. Chest X-ray showed miliary lung metastasis. (Figure 1A) Biopsy of the left breast mass confirmed hormone receptor (HR)–positive, HER2-negative, invasive ductal carcinoma.
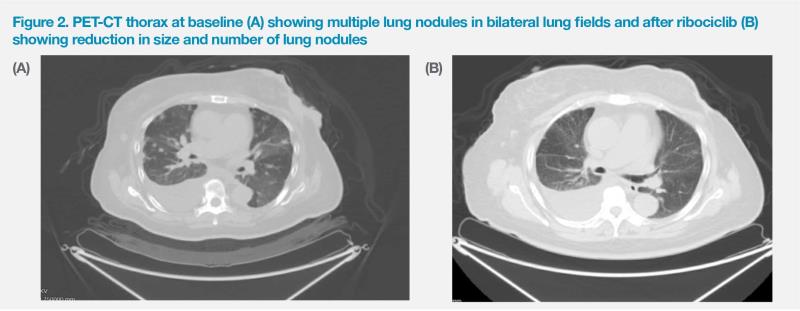
PET-CT scan in September 2022 showed the large, fungating and ulcerating left breast mass was around 10 cm in diameter with invasion to the skin and chest wall. Besides the fungating breast mass, there were extensive bone, multiple enlarged mediastinal lymph node and extensive lung metastases, with over 50 lesions in both lungs, each measuring approximately 1.5–2 cm in diameter. (Figure 2A)
The patient started initial treatment with an aromatase inhibitor (AI), letrozole, in September 2022. However, her shortness of breath persisted and she required home oxygen supplementation via nasal cannula at 2 L/min. In November 2022, she was referred to our department for further management. She was then started on the oral cyclin-dependent kinase (CDK) 4/6 inhibitor, ribociclib, at 600 mg daily in addition to letrozole. After completing the first cycle of ribociclib (ie, 21 consecutive days on treatment, followed by 7 days off treatment), the patient developed grade 3 neutropenia. Ribociclib was continued at a reduced dose of 400 mg daily from cycle 2 onwards. Her white blood cell and neutrophil counts were maintained without any additional granulocyte colony-stimulating factor.
The patient had a good and rapid response to treatment. Tumour shrinkage was evident within 1 month of starting ribociclib, which was accompanied by resolution of pain in the left breast. The fungating breast mass gradually decreased in size and flattened. The breast wound dried up with less bleeding and discharge 2 months after starting ribociclib. By February 2023 (4 months after commencing ribociclib) the patient no longer required supplemental oxygen and was able to walk unaided. Serial chest X-rays also confirmed improvement in her lung lesions. (Figure 1B) While the patient was chaperoned by family members to previous appointments, she was now able to come to the clinic by herself, as she no longer needed the bulky oxygen tank.
After starting ribociclib treatment, the patient’s cancer antigen 15-3 (CA 15-3) level improved from 16 U/mL (November 2022) to 4 U/mL (February 2023), while her serum carcinoembryonic antigen (CEA) level also decreased from 7.8 ng/mL to 3.7 ng/mL. In early April 2023, PET-CT scan confirmed partial response in the patient’s left breast mass (largest nodule measured approximately 4.5 cm) as well as in the lung and bone metastases. (Figure 2B) Her ECOG PS improved to 1 and she became able to independently perform activities of daily living without oxygen supplementation. She continued to tolerate the treatment well and received a total of six cycles of ribociclib plus letrozole. Treatment will continue indefinitely until disease progression or intolerance.
Discussion
Current international guidelines recommend the combination of a CDK4/6 inhibitor with endocrine therapy (ET), specifically AI, as first-line standard of care for postmenopausal patients with HR-positive, HER2-negative advanced breast cancer (ABC). This is due to improved progression-free survival (PFS) and overall survival (OS) as well as maintained or improved quality of life (QoL) associated with this combination.1,2
Among the possible CDK4/6 inhibitor combinations, only ribociclib received a category 1 recommendation from the National Cancer Comprehensive Network (NCCN) as first-line treatment for postmenopausal patients with HR-positive, HER2-negative ABC when used in combination with AI due to its associated OS benefits.1 This was demonstrated in the phase III, randomized, placebo-controlled MONALEESA-2 trial, which evaluated the efficacy and safety of ribociclib plus letrozole as first-line treatment in 668 postmenopausal women with HR-positive, HER2-negative recurrent or metastatic breast cancer who had not received previous systemic therapy for advanced disease.3,4
Patients in MONALEESA-2 received letrozole (2.5 mg QD) plus ribociclib (600 mg QD on a 3 weeks on, 1 week off schedule) or letrozole plus placebo.3 Primary analysis results showed that PFS was significantly longer in the ribociclib vs placebo group (median, 25.3 months vs 16.0 months; hazard ratio, 0.57; 95 percent confidence interval [CI], 0.46–0.70; p<0.001).3-5 After a median follow-up of 6.6 years, ribociclib yielded a significant OS benefit, with a median OS of 63.9 months vs 51.4 months in the ribociclib vs placebo group (hazard ratio, 0.76; 95 percent CI, 0.63–0.93; p=0.008).4 (Figure 3)
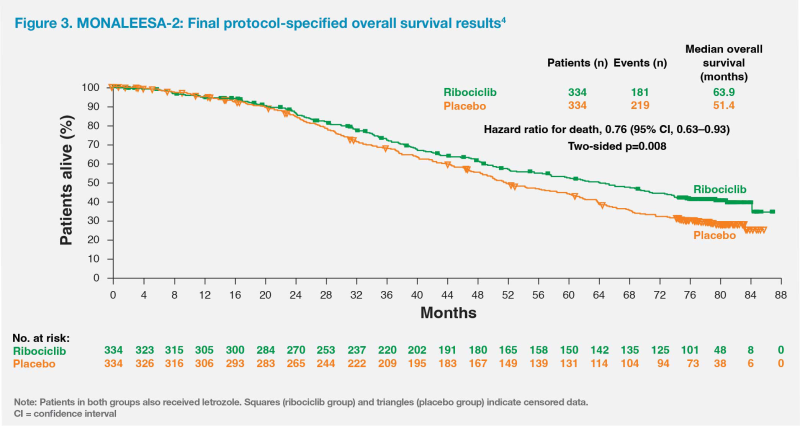
The survival curves showed that the OS benefit from ribociclib began to emerge at approximately 20 months, which remained consistent and continued to increase over time, with OS rates improving by 5.7 percent, 8.4 percent and 12.2 percent vs placebo after 4 years, 5 years and 6 years, respectively.6 The OS benefit was generally consistent across different patient subgroups.4
Adding ribociclib resulted in marked improvements in our patient’s symptoms and QoL, as evidenced by the rapid tumour shrinkage and healing of the fungating lesion as well as early resolution of breast pain and shortness of breath. This is consistent with the health-related QoL (HRQoL) outcome analysis of MONALEESA-2, which showed that ribociclib plus letrozole was associated with earlier and more durable tumour response as well as greater degree of tumour shrinkage and pain reduction vs placebo plus letrozole. In this subanalysis, decreased tumour size at 8 weeks was observed in a greater proportion of patients in the ribociclib (76 percent) vs placebo (67 percent) arm. In particular, more patients in the ribociclib (32 percent) vs placebo (17 percent) arm experienced best percentage change ≥60 percent. At 6 months, 37.2 percent of patients in the ribociclib arm achieved early response vs 23.2 percent in the placebo arm. The average pain reduction was also greater in patients who received ribociclib (26 percent) vs placebo (15 percent).7
Our patient had good response with early and considerable skin healing and tolerated her treatment well despite her advanced age. She only experienced grade 3 neutropenia, which resolved after ribociclib dose reduction from cycle 2 onwards. This is consistent with results of MONALEESA-2, where neutropenia was the most common grade 3/4 adverse event (AE) of special interest associated with ribociclib. Neutropenia could usually be improved with continuation of ribociclib after dose adjustment in the initial few cycles of treatment. There were no new safety signals in either treatment group at later analysis.4,8
Additional grade 3/4 AEs of special interest associated with ribociclib treatment in MONALEESA-2 included hepatobiliary toxic effects and QT rolongation.4 Although these AEs are known to be associated with ribociclib, they are not common in our clinical practice. Nevertheless, prevention and prompt management of these AEs may be achieved with careful monitoring. Liver function tests, for instance, should be performed every 2 weeks for the first two cycles, at the beginning of each subsequent four cycles, and as clinically indicated. A similar schedule may be followed for complete blood count and electrocardiography monitoring. Meanwhile, ribociclib should be avoided in patients with or at increased risk of developing QT prolongation, such as those with long QT syndrome and those with uncontrolled or significant cardiac disease.8 Although our patient had hypertension, T2DM and hyperlipidaemia, these comorbidities were well controlled, making ribociclib an appropriate treatment choice.
Consistent with current treatment recommendations and the survival and QoL benefits reported in a long-term clinical trial, this case report illustrates the effectiveness and tolerability of first-line ribociclib plus letrozole for an elderly postmenopausal woman with HR-positive, HER2-negative ABC and multiple comorbidities.