CD19-directed chimeric antigen receptor (CAR) T-cell therapy has shown impressive efficacy and manageable toxicity and has evolved as a new standard of care (SoC) for relapsed/refractory (R/R) B-cell lymphomas. At a Novartis-sponsored meeting organized by the Hong Kong Society of Haematology (HKSH), Professor Pier Luigi Zinzani of the Department of Medical and Surgical Sciences, University of Bologna, Italy, shared real-world data on CAR T-cell therapies, including from his institution, and compared them within the class as well as against clinical trial setting.
Real-world data concordant with clinical studies
Axicabtagene ciloleucel (axi-cel) and tisagenlecleucel (tisa-cel) are the first two CAR T-cell products to receive US FDA approval for treatment of diffuse large B-cell lymphoma (DLBCL) in the third or subsequent lines on the basis of the pivotal ZUMA-1 and JULIET studies, where axi-cel showed an objective response rate (ORR) of 82 percent and a complete response rate (CRR) of 54 percent, while tisa-cel showed the best overall response rate of 52 percent and a CRR of 40 percent. [N Engl J Med 2017;377:2531-2544; N Engl J Med 2019;380:45-56] As both ZUMA-1 and JULIET were relatively small phase II trials, longer-term data are being collected from real-world studies.
A non-interventional, prospective, longitudinal study in 1,159 adult patients with R/R DLBCL from the Center for International Blood and Marrow Transplant Research (CIBMTR) Registry who were treated with commercial tisa-cel in the US, Canada and Israel reported ORR of 59.5 percent, CRR of 44.5 percent, a progression-free survival (PFS) rate of 28.4 percent, and an overall survival (OS) rate of 43.6 percent at 24 months. [Landsburg DJ, et al, ASH 2021, presentation 656]
“The PFS and duration of response [DoR] curves were very similar, with much overlap between the JULIET-eligible and -ineligible [67.7 percent of patients] subsets, meaning that one can expect to cure a similar proportion of patients with tisa-cel in the real world as in the registration trial,” highlighted Zinzani.
According to single-centre experience in Bologna, Italy, a mixed population of patients with B-cell lymphomas (DLBCL, 75 percent) who received axi-cel (n=67) or tisa-cel (n=43) had an ORR of 80 percent and a CRR of 62 percent at a median follow-up of 21.3 months. Median PFS was 7.9 months, while OS was not reached. “After approximately 20 months, [we could see] a real plateau in PFS and OS curves, with several patients in continuous CR – whom we can consider cured,” shared Zinzani. [Zinzani PL, HKSH, October 2023]
Grade ≥3 cytokine-release syndrome (CRS) and immune-effector cell–associated neurotoxicity syndrome (ICANS) occurred in 6 percent and 15 percent of patients. Grade ≥3 anaemia, thrombocytopenia and neutropenia were reported in 48 percent, 42 percent and 96 percent of patients, respectively. A total of 31 and 54 infectious disease events were reported within and after 28 days from infusion, respectively. “Particular attention should be paid to late toxicities, especially infections, which are often responsible for late nonrelapse mortality [NRM],” advised Zinzani.
Tisa-cel vs axi-cel in the real world
Germany
A study of 356 adult R/R DLBCL patients enrolled in the German Registry for Stem Cell Transplantation (DRST) reported ORR/CRR of 74 percent/42 percent in the axi-cel cohort vs 53 percent/32 percent in the tisa-cel cohort (p<0.001). Kaplan-Meier–estimated respective PFS and OS rates at 12 months were 35 percent vs 24 percent and 55 percent vs 53 percent. While the PFS rate was significantly higher with axi-cel vs tisa-cel (plog-rank=0.015), there was no significant difference in OS rate. [Blood 2022;140:349-358]
Any-grade CRS was significantly more prevalent with axi-cel vs tisa-cel (81 percent vs 65 percent; p=0.0003), as was ICANS of any grade (44 percent vs 22 percent; p<0.0001). Grade ≥3 ICANS was reported in 16 percent vs 7 percent of patients (p=0.004). At 24 months, NRM occurred in 10.4 percent vs 3.5 percent of patients (p=0.032).
France
The retrospective French DESCAR-T registry study of 418 R/R DLBCL patients after ≥2 previous lines of treatment showed ORR/CRR of 80 percent/60 percent vs 66 percent/42 percent with axi-cel vs tisa-cel (p<0.001 for both). After a median follow-up of 11.7 months, the 1-year PFS rate was 46.6 percent vs 33.2 percent (p=0.0003). OS at 1 year was 63.5 percent vs 48.8 percent (p=0.0072). No significant difference was reported in DoR. [Nat Med 2022;28:2145-2154]
Of patients treated with axi-cel vs tisa-cel, 86.1 percent vs 75.6 percent experienced CRS. While grade 1–2 CRS was significantly more frequent with axi-cel vs tisa-cal (80.9 percent vs 66.5 percent; p<0.001), no significant difference was reported for grade ≥3 CRS (9.1 percent vs 5.3 percent; p=0.130). Both low-grade (34.9 percent vs 19.1 percent; p<0.001) and severe (13.9 percent vs 12.4 percent; p<0.001) ICANS were significantly more frequent with axi-cel vs tisa-cel.
Spain
A retrospective study in 261 patients with R/R DLBLC reported ORR/CRR of 60 percent/42 percent and median DoR of 12.5 months in the axi-cel group vs 54 percent/34 percent and 14.1 months in the tisa-cel group. The respective estimated 12- month rates of PFS (41 percent vs 33 percent; p=0.191) and OS (51 percent vs 47 percent; p=0.191) were not significantly different in the intention-to-treat population. [Haematologica 2023;108:110-121]
Frequency of all-grade (88 percent vs 73 percent; p=0.003) but not grade ≥3 (8 percent vs 6 percent; p=0.637) CRS was higher in the axi-cel vs tisa-cel group. Any-grade (42 percent vs 16 percent; p<0.001) and grade ≥3 (18 percent vs 5 percent; p=0.001) ICANS were significantly more frequent with axi-cel vs tisa-cel.
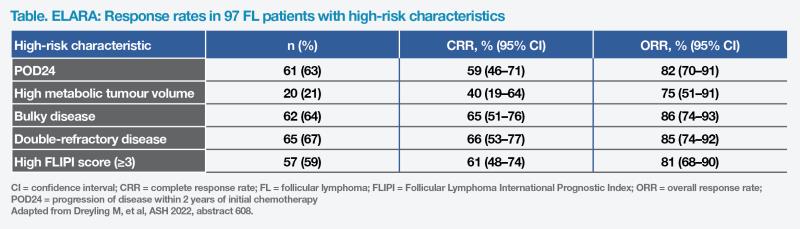
Tisa-cel in FL
ELARA is a single-arm trial investigating tisa-cel in treatment of 97 adult patients with R/R grade 1–3A follicular lymphoma (FL). After a prolonged median follow-up of 29 months, the high ORR (86 percent) and CRR (68 percent) were consistent with primary analysis. Median DoR, PFS and OS were not reached. [Dreyling M, et al, ASH 2022, abstract 608]
“Tisa-cel demonstrated high CRR in all subsets of high-risk patients, namely those with disease progression within 2 years of initial chemotherapy [POD24; 59 percent], bulky disease [65 percent], double-refractory disease [66 percent], or high FL International Prognostic Index [FLIPI] score [61 percent],” reported Zinzani. (Table) “Notably, there was no significant difference in DoR between patients with or without POD24.”
Grade 1–2 CRS was reported in 49 percent of patients, with no grade ≥3 events. ICANS occurred in 4 percent of patients, one of whom experienced grade ≥3 ICANS. Grade ≥3 Haematological disorders including cytopenias were reported in 44 percent of patients.