Interstitial lung disease (ILD) is a serious complication of rheumatoid arthritis (RA), contributing to increased morbidity and mortality. At a symposium during the 2023 International Conference of Chinese Rheumatologists (ICCR) held in Hong Kong, Professor Vanessa Smith of the Department of Internal Medicine, Ghent University, Ghent, Belgium, discussed the importance of early identification and management of patients with RA-ILD, and highlighted the value of the antifibrotic drug, nintedanib, in slowing lung function decline in progressive RAILD. Dr Shirley Chan of the Division of Rheumatology and Clinical Immunology, the University of Hong Kong, reported preliminary results from the RAISE study – a local study of RA-ILD screening and evaluation in high-risk RA patients.
RA-ILD: Epidemiology and clinical/histopathologic features
ILD is a frequent extra-articular manifestation of RA with a 10-year cumulative incidence of 11 percent. Whilst studies have reported prevalence rates of 4–68 percent, subclinical ILD may be detected in up to one-third of RA patients. [Arthritis Care Res (Hoboken) 2022;74:2042-2049; Chest 2009;136:1397-1405; Arch Intern Med 2008;168:159-166]
High-resolution (HR) CT scans from patients with RA-ILD commonly show inflammatory (eg, ground glass opacities [72.4 percent]) and fibrotic (eg, reticulation [93.1 percent], traction bronchiectasis [59.6 percent], honeycombing [53.4 percent]) features. The most frequent patterns seen on HRCT are usual interstitial pneumonia (UIP) and nonspecific interstitial pneumonia (NSIP), with the former pattern being associated with worse survival. [Respir Med 2018;134:24-30; Chest 2009;136:1397-1405; Respir Med 2017;126:100-104]
There is a scarcity of data on the risk of ILD development among RA patients treated with biologics or targeted synthetic disease-modifying antirheumatic drugs (tsDMARDs). One retrospective cohort study found tofacitinib to be the only biologic DMARD with a risk of ILD lower than adalimumab’s. [JAMA Netw Open 2023;6:e233640]
Importance of early RAILD detection
ILD is associated with an increased risk of death in patients with RA. A prospective, population-based study in Denmark (n=31,333) revealed 10-year mortality rates of 60.1 percent and 34.5 percent in RA patients with or without ILD, respectively. [Ann Rheum Dis 2017;76:1700-1706] Furthermore, a cross-sectional study of survival in patients with RA-ILD conducted in Spain (n=106) showed that delayed diagnosis of ILD (>24 months or 12–24 months from symptom onset vs <12 months) is associated with increased mortality (p=0.0051). [Sci Rep 2021;11:9184]
The American College of Rheumatology (ACR) has recently released clinical practice guidelines for screening, monitoring, and treatment of ILD in patients with systemic autoimmune rheumatic disease (SARD), including RA. Pulmonary function tests (PFTs; spirometry, lung volumes and diffusion capacity) and chest HRCT are conditionally recommended for ILD screening in patients with SARD at an increased risk of developing ILD. [https://rheumatology.org/ interstitial-lung-disease-guideline]
A survey assessing practice patterns of rheumatologists found that while 95 percent screen for respiratory symptoms in patients with RA, only 18 percent order PFTs in new RA patients with no respiratory symptoms. [Rheumatology (Oxford) 2022;61:1459-1467] “However, symptoms alone lack sensitivity for detection of RA-ILD,” cautioned Smith. “In one cohort of 63 RA patients diagnosed with RA-ILD by HRCT and PFTs, 90 percent lacked symptoms of significant dyspnoea or cough. In addition, PFTs alone lack specificity for detection of ILD, which underscores the importance of HRCT as a reliable clinical screening tool to identify RA-ILD.” [Clin Dev Immunol 2013;2013:406927; Thorax 2001;56:622-627]
The ACR guidelines also conditionally recommend against screening with 6-minute walk distance test, chest radiography, bronchoscopy, and ambulatory desaturation testing, and strongly recommend against screening with surgical lung biopsy in high-risk patients. [https://rheumatology.org/interstitial-lung-disease-guideline]
Practical screening algorithms
Various predictive risk scores using independent risk factors have been developed for identifying ILD in RA. In general, male gender, smoking, older age, older age at RA onset, presence of the MUC5B rs35705950 T allele, extra-articular manifestations, increased Disease Activity Score-28 (DAS-28), Clinical Disease Activity Index (CDAI) score >28, and erythrocyte sedimentation rate (ESR) >80 mm/hr are associated with increased risk of developing ILD. (Table) [Arthritis Rheumatol 2022;74:1755-1765; Reumatol Clin (Engl Ed) 2021;17:207-211; Reumatol Clin (Engl Ed) 2023;19:74-81; Rheumatol Int 2023;43:1515-1523]
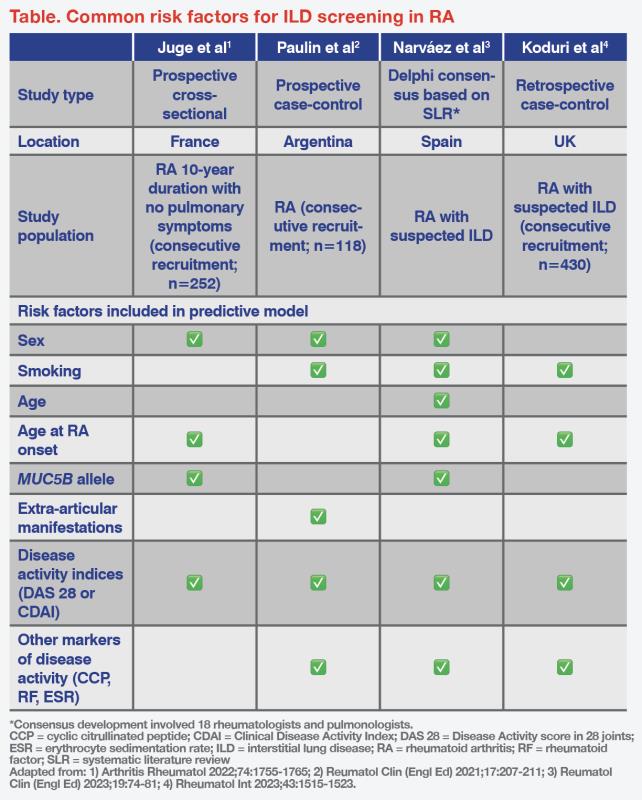
“In the RA-ILD screening criteria proposed by Narváez et al, the presence of ‘velcro-like’ crackles on auscultation, with or without respiratory symptoms, is an important indication for ILD screening in patients with RA,” highlighted Smith. “A screening algorithm based on ‘velcro’ crackles has demonstrated high diagnostic accuracy for RA-ILD [sensitivity, 93 percent; specificity, 77 percent].” [Reumatol Clin (Engl Ed) 2023;19:74-81; BMC Pulm Med 2019;19:111]
Close monitoring important for RA-ILD progression
A substantial proportion of patients with RA-ILD, including asymptomatic patients, develop progressive pulmonary fibrosis (PPF) characterized by worsening symptoms, decline in forced vital capacity (FVC)/carbon monoxide diffusing capacity (DLco), and/or radiological/HRCT evidence of progression. [Am J Respir Crit Care Med 2022;205:e18-e47; Respiration 2019;98:455-460; Arch Intern Med 2008;168:159-166]
“It is important to monitor for RA-ILD progression as it increases the risk of mortality,” noted Smith. In a population-based study (n=102), patients with progressive RA-ILD had 2-year and 5-year mortality rates of 28.6 percent and 55.0 percent, respectively, compared with 4.4 percent and 18.7 percent, respectively, for those with stable or improved RA-ILD. [Respiration 2019;98:455-460]
For monitoring of RA-ILD progression, the ACR guidelines suggest PFTs every 3–12 months for the first year, then less frequently once stable. In addition, ambulatory desaturation testing is suggested every 3–12 months, and chest HRCT is suggested when clinically indicated. [https://rheumatology.org/interstitial- lung-disease-guideline]
Antifibrotic therapy for progressive RA-ILD
The ACR guidelines conditionally recommend antifibrotics (eg, tyrosine kinase inhibitor nintedanib) and immunosuppressants, and conditionally recommend against use of long-term glucocorticoids in patients with progression of ILD on firstline therapy. [https://rheumatology.org/interstitial- lung-disease-guideline]
INBUILD trial
INBUILD was a randomized, double-blind, phase III trial that evaluated nintedanib in patients with progressive fibrosing ILDs other than idiopathic pulmonary fibrosis. All patients met ≥1 of the following criteria for ILD progression within the prior 24 months, despite management: a relative decline in FVC of ≥10 percent of predicted value; a relative decline in FVC of 5–<10 percent of predicted value and worsened respiratory symptoms or increased extent of fibrosis on HRCT; or worsened respiratory symptoms and increased extent of fibrosis. [N Engl J Med 2019;381:1718-1727]
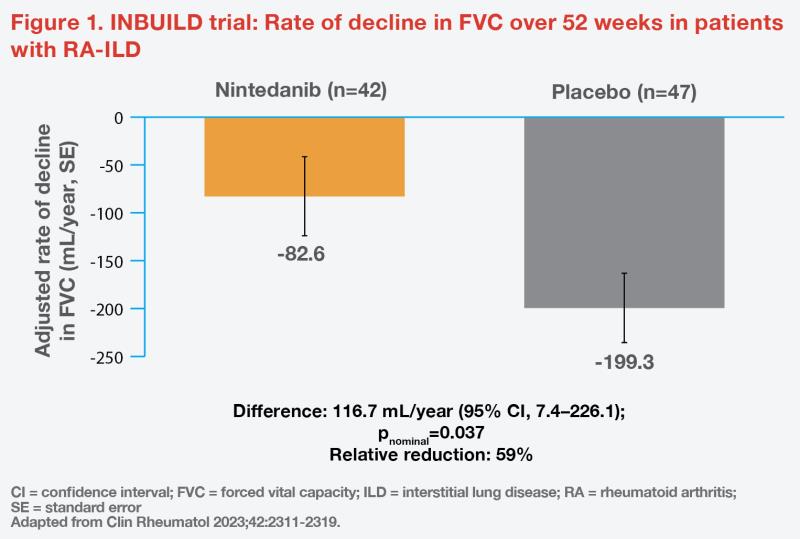
Of 663 participants in the trial, 89 (13.4 percent) had RA-ILD. In this subgroup, 88.8 percent were taking ≥1 immunomodulatory agents at baseline. Nintedanib reduced the rate of decline in FVC over 52 weeks by 59 percent vs placebo (-82.6 mL/year vs -199.3 mL/year; difference, 116.7 mL/year; 95 percent confidence interval [CI], 7.4–226.1; pnominal=0.037). (Figure 1) [Clin Rheumatol 2023;42:2311-2319]
Real-world data
A real-world, retrospective, cohort study (n=74) investigated the effectiveness and tolerability of antifibrotic medications (nintedanib and pirfenidone) in RA-ILD. Initiation of antifibrotic medication was found to significantly improve the estimated slope of percent predicted FVC (-0.3 percent/year after initiation vs -6.2 percent/year before antifibrotic initiation; p=0.03) after a median follow-up of 89 weeks. Male gender and heavy smoking were identified as risk factors for lung transplant and mortality. The most common adverse events in the study population were gastrointestinal (n=30), followed by disease progression (n=6). [Semin Arthritis Rheum 2023;64:152312]
HK RAISE study: RA-ILD screening and evaluation in high-risk RA patients
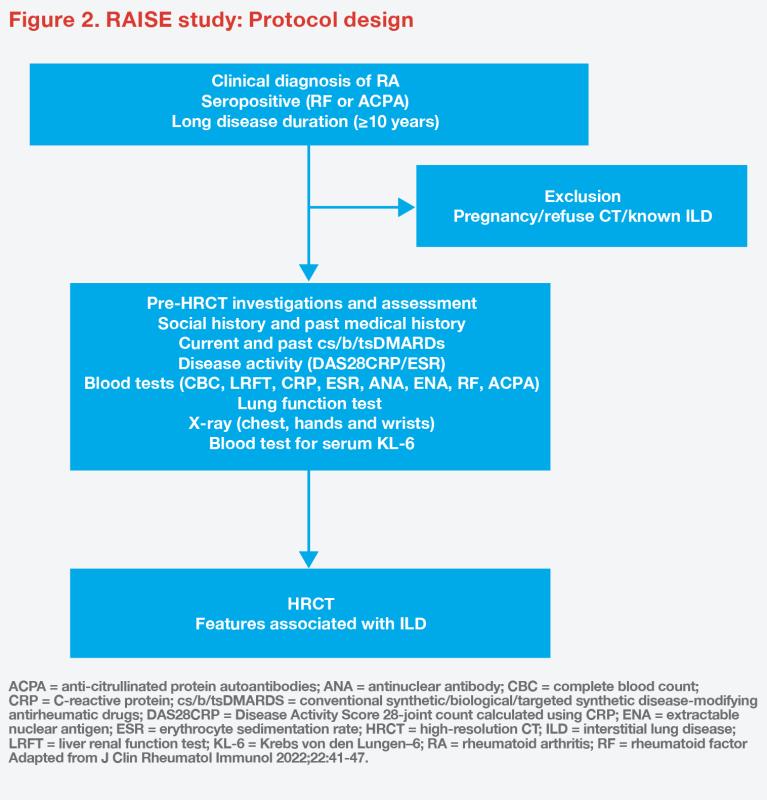
This is an ongoing cross-sectional, single-centre study designed with the primary objective to assess the prevalence of subclinical RA-ILD among high-risk RA patients in Hong Kong. Secondary objectives include identification of predictive factors associated with RAILD and examination of serum biomarkers, such as Krebs von den Lungen–6 (KL-6) levels. [J Clin Rheumatol Immunol 2022;22:41-47]
The study includes patients aged ≥18 years diagnosed with RA who are positive for rheumatoid factor (RF) or anti–cyclic citrullinated peptides (CCP), with a disease duration of ≥10 years. Baseline clinical assessments include disease activity, radiographs (chest, hands and wrists), PFTs, serum samples for protein biomarkers, thorax HRCT and lung ultrasound (blinded to HRCT findings). (Figure 2) The study aims to recruit a total of 110 patients.
Interim analysis
Between January and October 2023, 80 patients were enrolled and underwent HRCT. The median disease duration was 18.5 years. In this sample, only a minority were male (10 percent) or ever-smokers (10 percent). About half of the patients were on methotrexate (53 percent), and other medications included hydroxychloroquine (43 percent), sulphasalazine (21 percent), and biologics (25 percent).
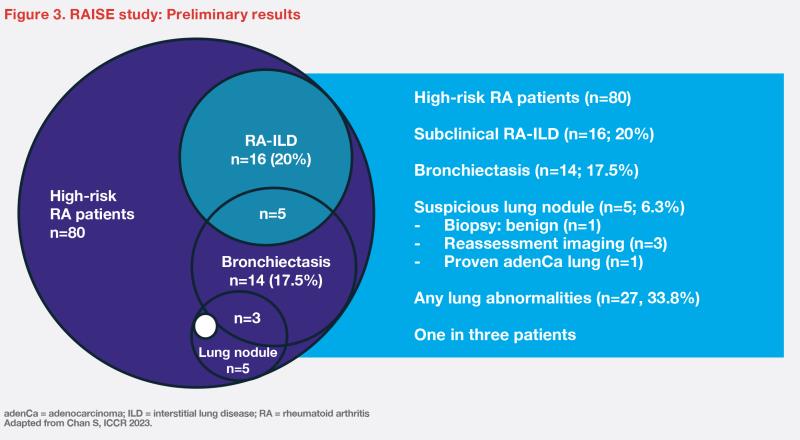
“At enrollment, only 6.7 percent of patients presented with FVC <75 percent,” commented Chan. “Subclinical RA-ILD was detected in 20 percent of patients. However, HRCT also revealed other lung abnormalities, such as bronchiectasis [17.5 percent] and suspicious lung nodules [6.3 percent], which prompted further testing.” (Figure 3) [Chan S, ICCR 2023]
“With appropriate screening, 33.8 percent of RA patients with long disease duration, who were asymptomatic [in terms of respiratory symptoms] and lacked traditional risk factors, were found to have lung abnormalities,” remarked Chan. “Apart from high levels of RF and anti-CCP, there were no other statistically significant clinical predictors for RA-ILD in our patient cohort.”
Summary
Development of ILD is common in patients with RA and is associated with high morbidity and mortality. Notably, the RAISE study in Hong Kong showed subclinical ILD in a substantial proportion of high-risk RA patients. Early identification and close monitoring of RA-ILD are essential to enable timely treatment to improve outcomes. In the INBUILD trial, nintedanib slowed the decline in FVC in patients with progressive fibrosing RA-ILD. Real-world evidence on antifibrotic therapy in RA-ILD further validates this beneficial effect on FVC decline.