Targeting the window of opportunity in management of hidradenitis suppurativa
Department of Dermatology
Kaohsiung Chang Gung Memorial Hospital
Kaohsiung, Taiwan

Hidradenitis suppurativa (HS) is a chronic inflammatory skin disease associated with systemic comorbidities and diminished health-related quality of life (HRQoL). At the Hong Kong Society of Dermatology and Venereology Annual Scientific Meeting 2023, Dr Han-Chi Tseng of the Department of Dermatology, Kaohsiung Chang Gung Memorial Hospital, Kaohsiung, Taiwan, reviewed the burden of HS, evidence for treatment with secukinumab, and the potential for early treatment initiation to change the disease course.
HRQoL burden
HS is characterized by recurrent inflammatory lesions in the apocrine gland–bearing skin of the axillary, inguinal and anogenital regions. [J Eur Acad Dermatol Venereol 2015;29:619-644] As HS progresses, patients develop painful nodules, scarring and pus-discharging sinus tracts and fistulae, which restrict movement and release malodourous secretions. [Nat Rev Dis Primers 2020;6:18]
“HS is associated with a marked reduction in HRQoL, and many patients become reclusive and socially withdrawn,” noted Tseng. Compared with other common skin disorders, HS sufferers have some of the lowest HRQoL, as indicated by Dermatology Life Quality Index scores. [J Eur Acad Dermatol Venereol 2013;27:473-478]
In addition, misdiagnosis is common, with a reported mean diagnostic delay of 7.2 years (vs 1.6 years for psoriasis), which leads to ineffective treatment, often causing disease progression. [N Engl J Med 2012;366:158-164; Br J Dermatol 2015;173:1546-1549]
“As the sites involved vary and early lesions are nonspecific, patients are often referred to different specialists, including dermatologists, gynaecologists, infectious disease specialists, general and plastic surgeons, immunologists, gastroenterologists, proctologists, and urologists, highlighting the need for education and multidisciplinary collaboration,” explained Tseng. “This failure to recognize HS and provide appropriate treatment significantly adds to patients’ distress and further impairs QoL.”
Treatment options for HS include anti-inflammatory drugs, antibiotics and, for severe disease, surgery. [J Eur Acad Dermatol Venereol 2015;29:619-644] Adalimumab, an anti–tumour necrosis factor antibody, has been approved for HS in some regions, but its reported efficacy varies, with clinical response achieved by approximately 50 percent of patients in phase III trials. [Br J Dermatol 2023;doi:10.1093/bjd/ljad345] Efficacy shrinkage has also been reported in some patients.
Phase III evidence for secukinumab in HS
Secukinumab, a monoclonal antibody against interleukin-17A, has been evaluated for HS treatment in two identical multicentre, randomized, placebo-controlled phase III trials – SUNSHINE (n=541) and SUNRISE (n=543). [Lancet 2023;401:747-761] Adults with moderate-to-severe HS were randomized 1:1:1 to receive subcutaneous (SC) secukinumab 300 mg every 2 weeks (SEC Q2W), SC secukinumab 300 mg every 4 weeks (SEC Q4W), or SC placebo. The primary endpoint was HS clinical response (HiSCR), defined as ≥50 percent decrease in abscess and inflammatory nodule count with no increase in the number of abscesses or draining fistulae at week 16 vs baseline. At week 16, patients in the placebo group were randomized 1:1 to receive SEC Q2W or SEC Q4W.
The primary endpoint was met in the SEC Q2W arm of both SUNSHINE and SUNRISE. In SUNSHINE, HiSCR was achieved in 45 percent vs 34 percent of patients in the SEC Q2W vs placebo arm (odds ratio [OR], 1.8; 95 percent confidence interval [CI], 1.1–2.7; p=0.007). In SUNRISE, HiSCR rates were 42 percent vs 31 percent for SEC Q2W vs placebo (OR, 1.6; 95 percent CI, 1.1–2.6; p=0.015).
The primary endpoint was also met with SEC Q4W in SUNRISE, with 46 percent vs 31 percent of patients in the SEC Q4W vs placebo arm achieving HiSCR (OR, 1.9; 95 percent CI, 1.2–3.0; p=0.0022), but not in SUNSHINE, where HiSCR was achieved by 42 percent vs 34 percent of patients in the respective groups (OR, 1.5; 95 percent CI, 1.0–2.3; p=0.042).
“HiSCR rates seen at week 16 were sustained through week 52 in both studies, with a trend for improvement over time,” highlighted Tseng. (Figure)
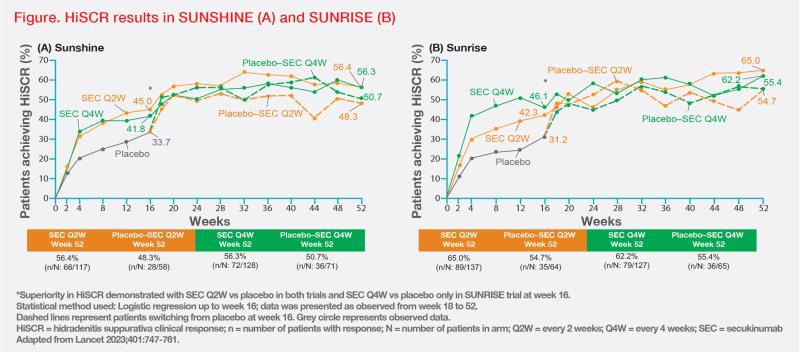
In both trials, patients in the SEC Q2W arm achieved significant improvements in all secondary endpoints vs placebo, namely abscess and nodule counts, pain, HRQoL, and disease flares (in SUNSHINE only).
Both secukinumab regimens showed good tolerability, with a similar pattern of adverse events (AEs) reported in the SEC Q2W, SEC Q4W and placebo arms. Common AEs reported up to week 16 were headache, nasopharyngitis and worsening of HS, while serious AEs were rare. Overall, secukinumab’s safety profile was consistent with that previously reported, with no new or unexpected safety findings detected.
Window of opportunity for early management
“The ‘window of opportunity’ concept refers to the importance of the early pathogenic phases in HS and other inflammatory skin conditions, as timely control of inflammation can help prevent irreversible tissue damage,” said Tseng. [J Invest Dermatol 2022;142:944-950; Br J Dermatol 2023;doi:10.1093/bjd/ljad345; Br J Dermatol 2021;184:10-11] Effective early treatment is necessary to avoid HS progression, surgery, and the debilitating physical and mental consequences of the condition.
Evidence supporting early treatment is available in psoriasis, for which secukinumab is approved. [Secukinumab Prescribing Information] In psoriasis studies of secukinumab, the time to relapse among patients who discontinued treatment was inversely correlated with duration of disease at baseline. [J Eur Acad Dermatol Venereol 2018;32:1930-1939]
Furthermore, efficacy of early secukinumab treatment has been validated in the STEPIn study (n=153), in which secukinumab was found to be superior to narrow-band ultraviolet B phototherapy for treatment of new-onset (≤12 months) moderate-to-severe plaque psoriasis (Psoriasis Area and Severity Index response rate at week 52, 91.1 percent vs 42.3 percent [OR, 16.3; 95 percent CI, 5.6–46.9; p<0.0001]). [J Eur Acad Dermatol Venereol 2023;37:1004-1016]
“The high and sustained skin clearance observed in psoriasis indicates that biologic treatment may be more effective if used early, and may support a change in treatment strategy for patients with new-onset moderate-to-severe disease – a finding likely relevant to HS too,” concluded Tseng.