Tumour-treating fields – a versatile treatment modality for multiple cancer types











Tumour-treating fields (TTFields) are a noninvasive cancer treatment modality, which first demonstrated clinical benefits in combination with chemotherapy in glioblastoma multiforme (GBM). At a webinar organized by the Hong Kong Cancer Therapy Society (HKCTS), experts discussed TTFields’ potential in other cancer types, including hepatocellular carcinoma (HCC) and pancreatic cancer, and the rationale for combining TTFields with immunotherapy.
TTFields mechanism of action
TTFields are noninvasive, low-intensity, intermediate-frequency, alternating electric fields generated by transducer arrays applied locoregionally to the skin proximal to the cancer site (eg, scalp for GBM patients), which are connected to a portable battery-operated device. [Sci Rep 2015;5:18046; Annu Int Conf IEEE Eng Med Biol Soc 2015;2015:6888-6891] “TTFields disrupt the localization and orientation of polar molecules, including tubulin subunits, which impacts mitotic spindle formation, leading to metaphase arrest, prolonged mitosis and, ultimately, death in dividing cancer cells. Their ubiquitous antimitotic mechanism of action suggests broad applicability,” said Dr Uri Weinberg, Chief Science at Novocure, Haifa, Israel. [Clin Cancer Res 2018;24:266-275]
TTFields can be frequency-tuned according to cancer cell histology and have so far received US FDA approval for GBM and malignant pleural mesothelioma (MPM).
Efficacy in combination with chemo or targeted therapy
“Exposure to TTFields creates a conditional vulnerability environment, which renders cells more susceptible to DNA-damaging agents or agents that interfere with DNA repair or replication fork maintenance,” explained Weinberg. [Transl Res 2020;217:33-46] Indeed, clinical data demonstrate enhanced efficacy of various standard-of-care (SoC) regimens, including chemotherapies, in combination with TTFields.
EF-14: TTFields + TMZ for GBM
The pivotal phase III, randomized, open-label EF-14 trial compared TTFields (applied ≥18 hours/day) plus maintenance temozolomide (TMZ) (intervention group) vs TMZ alone (control group) in 695 patients with newly diagnosed GBM who had completed concomitant chemoradiotherapy. [JAMA 2017;318:2306-2316]
After a median follow-up of 40 months, median progression-free survival (PFS) and median overall survival (OS) from randomization were 6.7 months and 20.9 months in the intervention group vs 4.0 months and 16.0 months in the control group (hazard ratio [HR] for PFS, 0.63;95 percent confidence interval [CI], 0.52 to 0.76; HR for OS, 0.63; 95 percent CI, 0.53 to 0.76; p<0.001 for both).
STELLAR: TTFields + platinum chemo for MPM
The STELLAR trial was a phase II, prospective, single-arm registration trial of TTFields used in combination with chemotherapy in first-line treatment of MPM. Patients (n=80) received continuous TTFields for ≥18 hours daily with concomitant pemetrexed and cisplatin or carboplatin for up to six cycles, followed by TTFields as maintenance treatment until disease progression. [Lancet Oncol 2019;20:1702-1709]
After a median follow-up of 12.5 months, median OS was 18.2 months, in contrast to the historical data of 12 months with chemotherapy alone. Among 72 patients evaluated for radiological response, disease control rate (DCR) at first CT scan was 97 percent with 40 percent of patients achieving partial response (PR) and 57 percent attaining stable disease (SD).
HEPANOVA: TTFields + sorafenib for HCC
TTFields applied at 150 kHz, alone and in combination with sorafenib, have been shown to reduce the proliferative potential of HCC cell lines in vitro and in vivo. This has formed the basis for the prospective, single-arm, historicalcontrol, phase II HEPANOVA EF-30 trial (NCT03606590) investigating the efficacy and safety of TTFields plus sorafenib for advanced HCC (n=25). [Davidi S, et al, ESMO GI 2020, abstract 261; Ann Oncol 2021;32(Suppl_3):S225]
“With 22.2 percent of patients having an Eastern Cooperative Oncology Group [ECOG] performance status of 2 and 51.8 percent of patients having a Child-Pugh score of B7/B8, this was a patient population with a rather poor prognosis, and is representative of this disease in clinical practice,” noted HEPANOVA coinvestigator, Professor Thomas Seufferlein of the Department of Internal Medicine at Ulm University, Germany. [Seufferlein T, HKCTS symposium, September 2021]
“Median duration of treatment was 10 weeks for TTFields and 9 weeks for sorafenib, which is quite short and indicative of the poor prognosis,” he continued. “Six patients died within 12 weeks of commencing treatment. However, among those who received TTFields with sorafenib for ≥12 weeks [n=11], the overall response rate [ORR] was 18 percent, with 18 percent of patients experiencing PR and another 73 percent DCR vs 76 percent DCR in the overall population [n=21]. ORR in the overall population was 9.5 percent – approximately double of what has historically been reported for sorafenib alone.” [J Clin Oncol 2015;33:559-566]
“Median PFS with TTFields plus sorafenib was 5.8 months despite the prognostically poor patient population. In the IMbrave150 trial, which included patients with a Child-Pugh score of A5/A6 and a better prognosis than those in HEPANOVA, the combination of atezolizumab with bevacizumab achieved a PFS of 6.8 months vs 4.3 months with sorafenib,” commented Seufferlein. [N Engl J Med 2020;382:1894-1905]
PANOVA: TTFields + chemo for pancreatic cancer
“Pancreatic cancer’s death toll is almost as high as its incidence,” said Seufferlein [World J Oncol 2019;10:10-27; Globocan 2020] “The treatment landscape is years behind most other cancer types, with chemotherapy remaining the SoC for the vast majority of patients. Since radiotherapy is ineffective and most identified not yet druggable, novel therapeutical approaches are of paramount importance for this ‘immune desert’ tumour.”
“HEPANOVA showed that TTFields can be applied safely and effectively to the abdominal region. Furthermore, pancreatic cancer tends to spread locally, meaning that the entire area at risk of metastasis can be covered by TTFields,” explained Seufferlein.
These findings as well as preliminary data from the pilot phase II PANOVA trial of TTFields in combination with gemcitabine (n=20) or gemcitabine plus nab-paclitaxel (n=20) for pancreatic adenocarcinoma, have formed the basis for PANOVA-3 (EF-27), an ongoing pivotal phase III, randomized, open-label study of TTFields concomitant with gemcitabine and nab-paclitaxel for frontline treatment of locally advanced pancreatic adenocarcinoma.
“In PANOVA, median PFS was 12.7 months, while median OS was not reached and the 1-year OS rate was 72 percent with TTFields, nab-paclitaxel and gemcitabine. In the historical trial, these values were 5.5 months, 8.5 months, and 35 percent, respectively”, reported Seufferlein. [Pancreatology 2019;19:64-67; N Engl J Med 2013;369:1691-1703]
Safety
Treatment with TTFields is typically well tolerated, with high compliance. GBM studies have demonstrated preserved quality of life. [JAMA Oncol 2018;4:495-504; J Clin Oncol 2021;39(Suppl_15):2055] The most common side effect associated with TTFields across all indications is skin toxicity, mostly localized dermatitis underneath the transducer arrays, which is experienced by 53–71 percent of patients, but most events are grade ½ and manageable with topical agents. [Front Oncol 2020;10:1045]
Combination with immunotherapy
“Effects of TTFields stretch beyond their antimitotic mechanism of action,” said Weinberg. One of the side effects being actively investigated is TTFields-mediated increase in antitumour immunity, which could serve as the basis for combining TTFields with immunotherapy.”
Immunogenic cell death primes the immune system through activation of dendritic cells (DCs), which elicits adaptive immune response. A study of ovarian cancer cells has shown that pretreatment with TTFields increases markers of immunogenic cell death, such as calreticulin, high-mobility group B1 and adenosine triphosphate, which facilitates cancer cell recognition and subsequent engulfment by dendritic cells. [Cancer Immunol Immunother 2020;69:1191-1204]
Additionally, in vivo studies demonstrate that combining TTFields with anti-PD-1 therapy results in significantly decreased tumour volume, increased tumour infiltration by interferon-y-producing T-cells and subsequent recruitment of antigen-presenting and CD8+ cytotoxic cells vs either treatment used alone. [Cancer Immunol Immunother 2020;69:1191-1204] “Collectively, these results serve as the basis for investigating the use of TTFields with immune checkpoint inhibitors [ICIs] in the ongoing lung cancer [LUNAR, KEYNOTE B36] and GBM [2-THE-TOP] trials,” shared Weinberg.
2-THE-TOP: TTFields + immunotherapy for GBM
“GBM is characterized by both local and systemic immunosuppression, which has meant multiple phase III disappointments for immunotherapies, such as ICIs and DC vaccines, in recent years,” said Dr David Trang of McKnight Brain Institute of the University of Florida College of Medicine, Gainesville, Florida, US. [Neuro Oncol 2019;21:348-359; Ther Clin Risk Manag 2018;14:1299-1313; JAMA Oncol 2020;6:1003-1010]
“However, treatment with TTFields activates the immune system against GBM cells. Data from our lab showed that TTFields-treated GBM cells had a significantly vs 4.3 percent for control; p=0.0032] of highly inflammatory micronuclei structures released into the cytoplasm as a result of TTFields-induced chromosomal instability. Nearly 40 percent of these micronuclei were colocalized with two upstream double-strand [ds] DNA sensors, AIM2 and cGAS, vs no co-localization in untreated cells. TTFields-activated micronucleids DNA sensor complexes led to induction of pyroptotic cell death and activation of STING pathway components, including pro-inflammatory cytokines,” reported Tran. (Figure) [Chen D, et al, AACR 2019, abstract 3280] “These results provide a strong rationale for combining TTFields with immunotherapy aimed at augmenting an antitumour immune response, such as ICIs.”
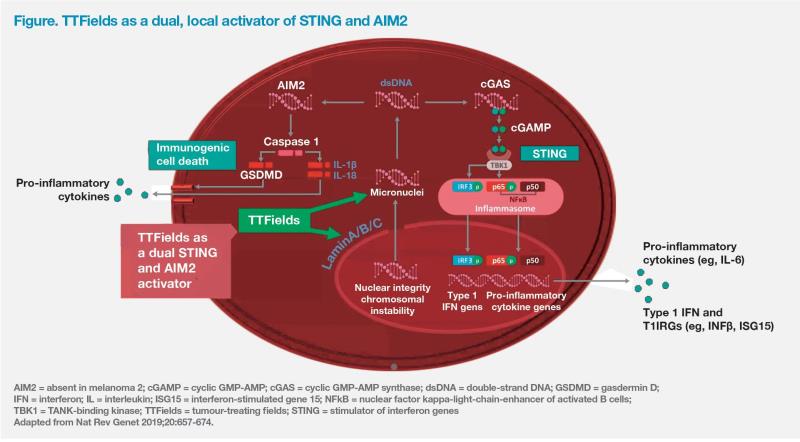
The ongoing, prospective, phase II single-arm 2-THE-TOP (NCT03405792) study is investigating whether adding the ICI pembrolizumab to TTFields plus maintenance TMZ will increase PFS in patients with newly diagnosed GBM vs historical control value attained with the current SoC, TTFields plus TMZ, in the EF-14 trial. The key secondary endpoints are adaptive immune activation by TTFields via type 1 interferon trajectory as well as augmentation of TTFields-initiated glioma-specific immune reaction by pembrolizumab.
“The study delays the addition of pembrolizumab to TTFields and TMZ in order to give us the opportunity to monitor the immune reaction to TTFields and TMZ alone and then assess any subsequent augmentation elaborated Tran. “Toxicity, tolerability and OS will also be evaluated. In addition, we will explore metabolomic signature changes in peripheral blood mononuclear cells, which will indicate immune activation following TTFields and pembrolizumab treatment, and whether there is a correlation between mutation burden in primary tumour samples and response to the triple regimen.”