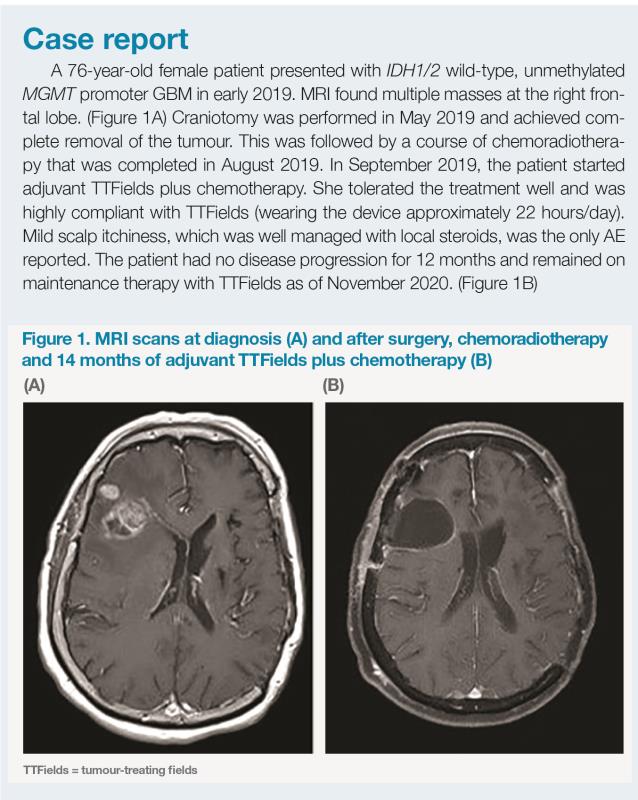
Tumour-treating fields: New treatment modality for glioblastoma




Glioblastoma multiforme (GBM) is a cancer with a notoriously poor prognosis. Tumour-treating fields (TTFields), a novel noninvasive modality, is the first treatment to demonstrate a survival benefit in GBM patients since 2005. At the Hong Kong College of Radiologists’ 28th Annual Scientific Meeting (HKCR 2020), Dr Tai-Chung Lam of the University of Hong Kong presented international and local clinical trial data on TTFields and shared a case on successful use of TTFields in an elderly patient.
TTFields for newly diagnosed GBM
TTFields inhibit rapidly dividing tumour cells by delivering low-intensity (1–3 V/cm), intermediate-frequency (200 kHz), bidirectional, alternating electric fields via transducer arrays that are applied to the scalp and are connected to a portable device. [NovoTTF-200A System Instructions For Use]
The pivotal phase III, randomized, open-label EF-14 trial compared TTFields (applied ≥18 hours/day) plus adjuvant temozolomide (TMZ) (intervention group) vs TMZ alone (control group) in 695 patients with newly diagnosed GBM who had completed concomitant chemoradiotherapy. In the intervention group, TTFields could be continued until the second radiologic progression or for a maximum of 24 months. [JAMA 2017;318:2306-2316]
After a median follow-up of 40 months, median progression-free survival (PFS) and median overall survival (OS) from randomization were 6.7 months and 20.9 months in the intervention group vs 4.0 months and 16.0 months in the control group (hazard ratio [HR] for PFS, 0.63; 95 percent confidence interval [CI], 0.52 to 0.76; HR for OS, 0.63; 95 percent CI, 0.53 to 0.76; p<0.001 for both).
“This significant improvement in PFS of about 3 months, demonstrated in a phase III clinical trial, has been long awaited since the approval of the Stupp regimen in 2005. The results were particularly encouraging as the PFS improvement translated to a consistent and sustained OS improvement for up to 5 years after randomization,” said Lam. “Moreover, TTFields are very well tolerated because they are applied locally to the cranium. No increases in grade ≥3 adverse events [AEs] and no systemic AEs related to TTFields have been reported.”
Positive data in recurrent GBM
In EF-11, a phase III pivotal randomized trial, TTFields monotherapy was compared with physician’s choice of chemotherapy in 237 patients with recurrent GBM. [Eur J Cancer 2012;48:2192-2202] “This patient population was very difficult to treat because nearly 90 percent had disease recurrence more than once and almost 20 percent had already been treated with bevacizumab at baseline. While TTFields demonstrated similar 1-year and 2-year OS rates vs chemotherapy, it was remarkable that about 9 percent of patients treated with TTFields were long-term survivors [beyond 3 years], since OS from GBM recurrence is commonly short [7–9 months on average],” pointed out Lam.
“More importantly, safety and quality of life analyses both favoured TTFields. Unlike chemotherapy, TTFields were not associated with haematological AEs or other systemic toxicities. There was also a lower incidence of severe AEs in the TTFields vs chemotherapy group [6 percent vs 16 percent; p=0.022],” Lam said.
TTFields improved treatment outcomes in elderly GBM patients
More than half of GBM patients are ≥65 years old at diagnosis, but elderly patients often receive less intensive therapy and usually have a worse prognosis, with a median OS of only about 6 months, according to real-world data. [J Neurol Sci 2017;380:250-255]
“TTFields are shown to be very well tolerated and highly beneficial for elderly patients with newly diagnosed GBM. In a subgroup analysis of EF-14, both PFS [median, 6.5 months vs 3.9 months; HR, 0.47; 95 percent CI, 0.30 to 0.74; p<0.0236] and OS [median, 17.4 months vs 13.7 months; HR, 0.51; 95 percent CI, 0.33 to 0.77; p<0.020] were prolonged in elderly patients who received TTFields plus TMZ vs TMZ alone. Furthermore, a real-world study [n=11,029] showed no increase in risk of skin reactions or falls and fractures in elderly patients treated with TTFields vs other age groups. Notably, elderly patients had the lowest incidence of TTFields-related tingling, headache and under-array heat sensation compared with patients in other age groups,” highlighted Lam. [J Clin Oncol 2020;38(Suppl 15):e24019; J Neurooncol 2020;148:489-500]
Adjuvant TTFields in GBM: A HK territory-wide cohort study
“In December 2018, Hong Kong became the first city in Asia outside Japan to use TTFields in GBM treatment. To date, 34 Hong Kong patients with GBM or high-grade glioma have received treatment with TTFields. Fourteen of these patients, who have completed the Stupp regimen for GBM, have been enrolled in a prospective, observational cohort study,” shared Lam.
“Patients received first-line adjuvant TTFields with six cycles of TMZ followed by TTFields maintenance. They were allowed to choose whether to continue with TTFields in case of progression, and in this 14-patient cohort, all patients who had progression chose to continue TTFields. Preliminary results showed an excellent survival benefit with TTFields: median PFS was 13.6 months and 1-year OS rate was nearly 90 percent, which seemed to be higher than that in EF-14 [73 percent]. Although these observational data are not mature and not meant to be compared directly with EF-14, they appear to be very promising,” commented Lam.
“Treatment with TTFields was also well tolerated, with high compliance [median use, 20.4 hours/day] and demonstrated excellent, well preserved quality of life among the participants,” added Lam, referring to as yet unpublished data. [Lam TC, et al, HKCR 2020]
Conclusion
TTFields are a new, effective and well-tolerated treatment modality for GBM. Randomized clinical trials and early local data have demonstrated clear PFS and OS benefits with TTFields in patients with newly diagnosed GBM, including the elderly. Evidence also suggests that applying TTFields beyond disease progression may continue to benefit GBM patients.