Use of a PCSK9 inhibitor in a patient with established ASCVD
Specialist in Cardiology
Hong Kong Sanatorium & Hospital
Hong Kong
History, presentation and treatment
A 54-year-old nonsmoking Australian male with a known history of hypertension presented with chest discomfort in September 2019. His LDL-cholesterol (LDL-C) level was 2.2 mmol/L. Coronary CT angiography in 2011 indicated mild stenosis at the left anterior descending (LAD) and left circumflex (LCX) arteries. However, assessment in September 2019 revealed stenosis progression, with 50–70 percent stenosis in the proximal LAD (p-LAD) artery and 50 percent stenosis in the proximal LCX (p-LCX) artery. Pulmonary CT angiography showed segmental pulmonary embolism (PE). Ultrasonography showed left lower limb deep vein thrombosis (DVT).
Treatment of venous thromboembolism with a direct oral anticoagulant (DOAC), apixaban 10 mg BID PO for 1 week followed by 5 mg BID PO, was commenced in September 2019. On the basis of his documented atherosclerotic cardiovascular disease (ASCVD), statin-naïve status and his preference for a low-intensity regimen, the patient was also prescribed simvastatin 20 mg daily. Based on the 2019 European Society of Cardiology (ESC)/European Atherosclerosis Society (EAS) guidelines, his LDL-C goal was <1.4 mmol/L and ≥50 percent LDL-C reduction from baseline due to the presence of two major vessels with ≥50 percent stenosis on CT.1
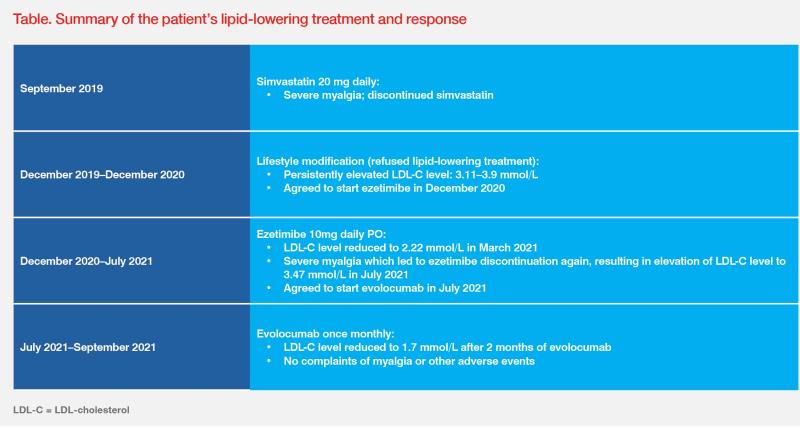
However, he experienced severe myalgia and discontinued simvastatin prior to the 3-month follow-up visit in December 2019. (Table)
Discussion
ASCVD remains a leading cause of morbidity and mortality globally.4 In China, ASCVD accounted for >40 percent of deaths in 2019.5 Cumulative exposure of the arterial wall to high levels of LDL-C is pivotal in the development and progression of ASCVD.6,7 In two meta-analyses, the Cholesterol Treatment Trialists’ (CTT) collaborators noted that greater LDL-C reductions were associated with significantly greater proportional reductions of major vascular events.8 With each 1 mmol/L reduction of LDL-C, the annual rate of these major vascular events was reduced by more than a fifth.9 This body of evidence confirms the general principle that “lower is better” for LDL-C levels.1,10
The guideline-recommended treatment goal for our patient was in accordance with the 2019 ESC/ EAS guidelines for management of dyslipidaemias, which recommended intensive reduction of LDL-C to <1.4 mmol/L and ≥50 percent LDL-C reduction from baseline for primary or secondary prevention of ASCVD in very-high-risk patients (ie, patients with documented multivessel CVD on imaging).1 This guideline-recommended strategy to substantially reduce LDL-C has been demonstrated through PCSK9 inhibition with the monoclonal antibody, evolocumab. In the pivotal FOURIER trial, 27,564 patients with ASCVD and LDL-C ≥1.8 mmol/L despite statin treatment were randomized 1:1 to receive evolocumab 140 mg Q2W or 420 mg QM or placebo. At a median of 2.2 years of follow-up, substantial reduction of LDL-C was observed in a significantly greater proportion of patients in the evolocumab vs placebo group (LDL-C reduced to ≤1.8 mmol/L, 87 percent vs 18 percent; LDL-C ≤1.0 mmol/L, 67 percent vs 0.5 percent; LDL-C ≤0.65 mmol/L, 42 percent vs <0.1 percent; p<0.001 for all). Evolocumab significantly reduced the risk of the primary composite endpoint of cardiovascular (CV) death, MI, stroke, hospitalization for unstable angina, or coronary revascularization vs placebo (hazard ratio [HR], 0.85; 95 percent confidence interval [CI], 0.79–0.92; p<0.001). No significant difference in AEs was noted between groups.11
Long-term efficacy and safety of evolocumab were further explored in an open-label, long-term extension study of FOURIER. In FOURIER-OLE, 6,635 eligible patients from FOURIER received evolocumab 140 mg Q2W or 420 mg QM regardless of their original group allocation. After a median follow-up of 5.0 years, patients in the FOURIER-evolocumab group had a significantly lower risk of the primary efficacy endpoint vs the FOURIER-placebo group (HR, 0.85; 95 percent CI, 0.75–0.96; p=0.008). Consistent reduction in LDL-C was sustained over long-term follow-up amongst patients in the FOURIER-evolocumab group. No increase in the incidence of AEs of interest was observed over time.12
Lifestyle interventions, including a healthy diet and increase in physical activity, are pivotal in the overall strategy of lipid management.5 However, lifestyle changes alone exert only a small effect on LDL-C levels.1 For very-high-risk patients with untreated LDL-C levels ≥1.8 mmol/L, the ESC/EAS guidelines suggest an integrated approach of lifestyle interventions and concomitant drug therapy.1 As demonstrated in our statinand ezetimibe-intolerant patient who failed to reach his LDL-C goal through lifestyle management, he responded well to evolocumab, showing significant improvement in LDL-C levels, with good treatment adherence and tolerance.
Evolocumab is indicated in patients with established ASCVD to reduce CV risk by lowering LDL-C levels, as an adjunct to correction of other risk factors, either as monotherapy or in combination with non-statin lipid-lowering therapies in cases of statin intolerance, such as our patient.3 The proven long-term efficacy and tolerability profile of evolocumab remain pivotal in the targeted approach to improving lipid profile of patients at very high CV risk, such as our patient, by reducing LDL-C levels to a maximum possible extent.