Use of radioligand therapy in a patient with metastatic GEP-NET
Specialist in Clinical Oncology
Private practice
Hong Kong

Presentation and investigations
A 64-year-old male with good past health presented in March 2019 with 2 days’ history of abdominal pain, which subsided on its own.
Ultrasound of the abdomen showed multiple liver metastases. Fluorodeoxyglucose (FDG) PET-CT scan in April 2019 showed >10 liver metastases (SUVmax, 13.3–24.1), along with hypermetabolic lesions in the pancreatic body (2.1 cm x 0.8 cm; SUVmax, 4.4) and tail (1.2 cm x 0.9 cm; SUVmax, 6.3), abdominal metastases, and suspected bone metastases. Biopsy revealed grade 2, nonfunctioning pancreatic neuroendocrine tumour (pNET; mitosis count, <2 per 10 HPF; Ki67 index, 3 percent), which was positive for cytokeratin AE1/AE3, synaptophysin, chromogranin A (CgA), and CD56. His baseline serum CgA and alpha-fetoprotein (AFP) levels were 450 ng/mL and 349.9 ng/mL, respectively, while Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 0.
Treatment and response
The patient was started on prolonged-release lanreotide 120 mg Q4W plus everolimus 10 mg QD in September 2019. However, he defaulted treatment after 2 months due to insurance issues. In March 2020, octreotide scan revealed disease progression, with numerous marked nodule uptakes in the liver, enlarged left para-aortic lymph node (LN) and ill-defined mild uptake at the pancreatic tail. FDG PET-CT scan in June 2020 showed numerous confluent liver metastases replacing more than 50 percent of the normal liver parenchyma. Treatment with lanreotide and everolimus was resumed in August 2020, resulting in stable disease observed on PET-CT in December 2020. After 8 months of treatment, everolimus was discontinued due to development of diabetes and hyperlipidaemia. Gallium (68Ga)-dotatate + FDG PET-CT in May 2021 showed stable disease in general, and a new nonspecific FDG uptake over the prostate.
In October 2021, 68Ga-dotatate + prostate-specific membrane antigen (PSMA) + FDG PET-CT scan revealed slow growth of liver, LN and bone lesions and solitary PSMA uptake over the prostate gland. Prostate-specific antigen (PSA) level was elevated to 19 ng/mL and it was consistent with a diagnosis of prostate cancer. Prostatectomy was performed on 17 December 2021, with pathologic findings of Gleason score 8 (4+4), pT2N0, margin-negative prostate cancer. PSA level decreased to 0.7 ng/mL in January 2022.
In April 2022, the patient experienced radiographic disease progression, as evidenced by multiple new bone lesions (SUVmax, 2.9–10.7) in T2 and T8–T9, along with enlargement of existing bone metastases, conglomerated liver mass in the superior right lobe (156 mm x 103 mm), and liver masses in the lateral right lobe (120 mm x 84 mm) and segment IV a/b (size: 62 mm x 58 mm).
One of the sclerotic bone lesions in the left inferior pubic ramus demonstrated higher PSMA and FDG avidity than that of 68Ga-dotatate, suggesting the possibility of single bone metastasis from the primary prostate cancer in the context of rising PSA level (2.65 ng/mL). The suspected bone metastasis was effectively treated with hormone therapy (leuprorelin acetate 30 mg Q6M) plus stereotactic body radiotherapy (SBRT).
Given multiple new bone metastases, radioligand therapy (RLT), lutetium (177Lu) oxodotreotide (177Lu-dotatate), was initiated for his pNET, with the recommended 4 cycles of treatment administered in November 2022, February 2023, April 2023, and June 2023.1 He tolerated the treatment well without apparent adverse events (AEs) or renal toxicity.
Follow-up 68Ga-dotatate PET-CT scan in August 2023 showed remarkable shrinkage of the conglomerated liver lesion (from 160 mm to 120 mm), bilobar liver metastases, and tumour in the pancreatic tail (from 2 cm to 1.3 cm), and general improvement in bone metastases with SUVmax dropping by 80 percent on average. (Figure 1)
Last seen in October 2023 (1 year after treatment with 177Lu-dotatate), the patient remained well without disease progression. He was asymptomatic and fully active, with an ECOG PS of 0.
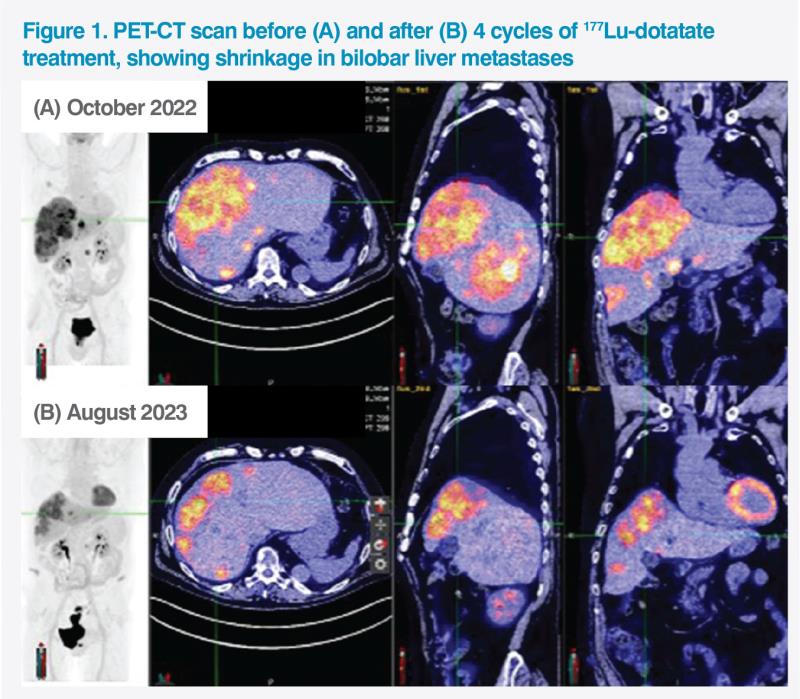
Discussion
Gastroenteropancreatic NETs (GEP-NETs) are rare tumours originating from the endocrine system in the gastrointestinal (GI) tract and pancreas, with varying clinical manifestations. Some patients present with symptoms due to hormonal hypersecretion (eg, flushing, diarrhoea, abdominal pain, heart disease) or tumour bulk, while others remain asymptomatic for years, particularly if the tumours are nonfunctional (nonsecretory).2 In the case of our patient with nonfunctional, metastatic pNET, the only presenting symptom was abdominal pain, which resolved on its own after 2 days.
High concentration of somatostatin receptors (SSTRs) is present in 70–95 percent of well-differentiated (grade 1–2) GEP-NETs, except for insulinoma.2 Somatostatin analogues (SSAs; eg, lanreotide, octreotide) represent first-line treatment options for most patients with well-differentiated GEP-NETs.3
As mass-effect and hormonal symptoms may develop during the course of the disease, follow-up investigations for patients with GEP-NETs should include symptom and biomarker (eg, CgA) monitoring.3 Changes in CgA may not necessarily indicate the need for treatment change, but instead the need for frequent monitoring. During first-line treatment, regular imaging is essential for evaluating tumour evolution and treatment response. According to the American Society of Clinical Oncology (ASCO) Guidelines, follow-up imaging should be performed every 3–6 months in patients with well-differentiated metastatic GEP-NETs.4
When unequivocal disease progression (eg, appearance of new lesion[s]) is documented on first-line treatment, 177Lu-dotatate is a second-line treatment option for SSTR-positive GEPNETs recommended in the European Society for Medical Oncology (ESMO), National Comprehensive Cancer Network (NCCN) and European Neuroendocrine Tumor Society (ENETS) guidelines.3,5,6
This recommendation is based on the results of the phase III NETTER-1 trial, which evaluated the efficacy of 177Lu-dotatate and long-acting octreotide 30 mg Q4W in metastatic well-differentiated mid-gut NETs (n=229). After a median follow-up of 14 months, 177Lu-dotatate with long-acting octreotide significantly lowered the risk of disease progression or death by 79 percent vs high-dose long-acting octreotide 60 mg Q4W (median progression-free survival [PFS], not reached vs 8.4 months; hazard ratio [HR], 0.21; 95 percent confidence interval [CI], 0.13–0.33; p<0.001).3,7 Treatment with 177Lu-dotatate also led to an 11.7-month improvement in median overall survival (OS) vs high-dose octreotide (48 months vs 36.3 months; HR, 0.84; 95 percent CI, 0.60–1.17; two-sided p=0.30), which is considered clinically relevant.8
An analysis of NETTER-1 showed that 177Lu-dotatate significantly prolonged time to deterioration in health-related quality of life (QoL) for domains including global health status (HR, 0.406), physical functioning (HR, 0.518), role functioning (HR, 0.580), fatigue (HR, 0.621), pain (HR, 0.566), diarrhoea (HR, 0.473), disease-related worries (HR, 0.572), and body image (HR, 0.425) vs high-dose octreotide.9 Although NETTER-1 focused on midgut NETs, similar results were noted in our patient with pNET, who maintained good QoL and remained fully active throughout treatment, without any symptoms or apparent AEs.
Second-line use of 177Lu-dotatate in unresectable progressive pNETs, as in our patient, was supported by positive results from the international, retrospective NETTER-R (n=110) and phase II OCLURANDOM (n=84) studies.10,11 Notably, our patient achieved remarkable tumour shrinkage with 177Lu-dotatate, which is uncommon in pNETs, as both targeted therapies (ie, sunitinib and everolimus) and SSAs are associated with low response rates.2,12 In the NETTER-R study, objective response rate was 40.3 percent in 177Lu-dotatate–treated patients with pNETs.10 In OCLURANDOM, after a median follow-up of 40 months, 177Lu-dotatate improved 12-month PFS rate vs sunitinib (80 percent vs 42 percent; median PFS, 20.7 months vs 11.0 months) without new safety concerns.11 (Figure 2)
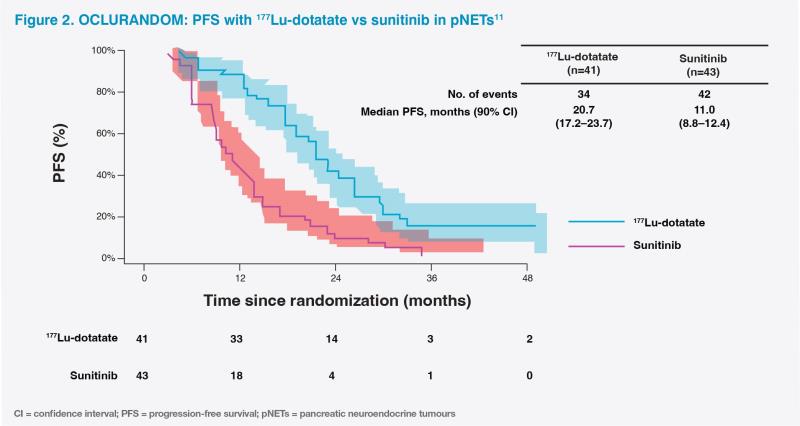
177Lu-dotatate has a generally favourable safety profile, with limited long-term toxicities.8,13 At the time of final OS analysis of NETTER-1, only 7 of 111 patients (6 percent) in the 177Lu-dotatate arm experienced grade ≥3 treatment-related serious AEs. No new cases of myelodysplastic syndrome or acute myeloid leukaemia were reported during long-term follow-up.8
Of note, 177Lu-dotatate, when administered concomitantly with a renoprotective agent, showed no apparent long-term renal toxic effects in NETTER-1.7,8 The phase I/II Erasmus trial also showed no therapy-related long-term renal or hepatic failure among 610 patients treated with 177Lu-dotatate, after a median follow-up of 64 months.13
Apart from 177Lu-dotatate, established second-line treatment options for GEP-NETs include everolimus, sunitinib (for pNETs), cytotoxic chemotherapy, and dose escalation of SSAs.3,4 NETTER-1 provided evidence to challenge the role of higher-dose SSAs, showing that RLT was more efficacious in mid-gut NETs.7 Although everolimus, sunitinib and chemotherapy can be used in the presence or absence of SSTR expression, these agents carry significant off-target effects.14 For instance, in our patient, treatment with everolimus led to evident metabolic disturbances. In contrast, the RLT 177Lu-dotatate delivers highly localized radiation to tissues expressing SSTRs, which are commonly found in NETs.1,2 As an SSTR-targeted treatment, 177Lu-dotatate is indicated in cases with tumour uptake as high as normal liver uptake on SSTR imaging, which is suggestive of 177Lu-dotatate’s subsequent antitumour effect.1
Careful assessment of patients’ renal, hepatic and bone marrow functions is critical before and during treatment with 177Lu-dotatate.1 Adequate nepphroprotection in the form of a combined solution of L-lysine HCl and L-arginine HCl should be provided.15,16
In summary, 177Lu-dotatate represents an efficacious and well-tolerated treatment option for metastatic or unresectable, progressive, well-differentiated GEP-NETs that overexpress SSTRs.3,4 Our patient’s positive experience of good tumour responses, PFS of ≥12 months and improved QoL replicate clinical evidence from randomized controlled trials and real-world studies.7–11,13