Use of tumour-treating fields for glioblastoma: Local experience








Case 1
History and presentation
A 55-year-old male experienced a sudden episode of headache and vomiting in May 2018. He subsequently developed generalized seizures that spontaneously resolved. Upon hospitalization, he had global aphasia and was unable to follow simple commands.
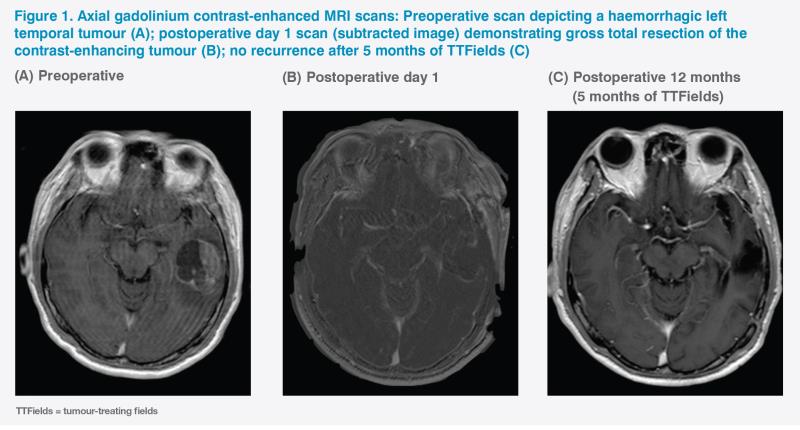
A brain MRI revealed a left middle temporal gyrus intra-axial lesion that exhibited considerable mass effect. (Figure 1A) The preoperative diagnosis was a left temporal high-grade glioma with intratumoural haemorrhage.
Treatment and response
An emergency craniotomy for gross total tumour resection was performed under general anaesthesia uneventfully. (Figure 1B) The patient fully recovered his language capabilities and was discharged on postoperative day 3 with no focal neurological deficit. Upon discharge, he had a Karnofsky performance score (KPS) of 90 and an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 1. The final histopathological diagnosis was glioblastoma multiforme (GBM) with wild-type IDH-1 and unmethylated MGMT promoter, which was considered unfavourable for overall survival (OS).
The patient received concomitant temozolomide (TMZ) chemoradiotherapy with a total of 60 Gy of radiation given over 30 fractions. After three adjuvant cycles of TMZ (i.e. 6 months following initial diagnosis), he began tumor-treating fields (TTFields) therapy. Both the patient and his main caregiver found TTFields tolerable. The patient received a total of six cycles of TMZ and declined further chemotherapy. Six-monthly MRI scans revealed no tumour recurrence. (Figure 1C)
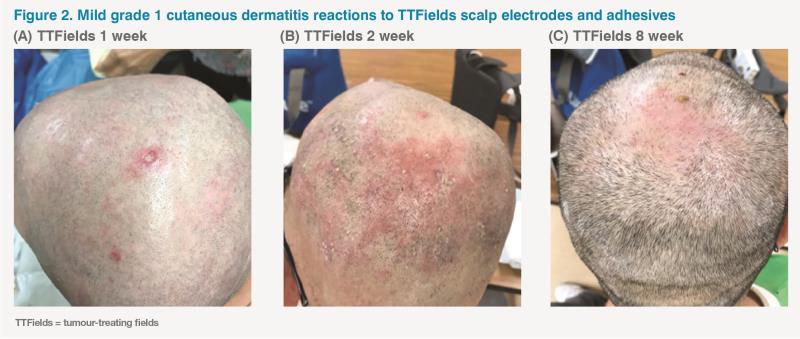
The patient’s overall compliance with TTFields was 75 percent (18 hours a day with the device attached to the scalp and active). The only adverse event (AE) was grade 1 scalp dermatitis, which did not necessitate disruption of TTFields therapy. (Figure 2) During this period, the patient’s PS, global quality of life (QoL) and symptom severity remained generally acceptable.
The patient’s disease progressed after 12 months of TTFields therapy (with TTFields given as sole treatment for 9 months) (i.e, progression-free survival [PFS], 18 months). A repeat craniotomy was performed under general anaesthesia, and MRI on postoperative day 1 showed no residual contrast-enhancing lesion. The histological diagnosis remained GBM. The patient had no additional focal neurological deficit, having a KPS of 90 and an ECOG PS of 0. His scalp wound healed well and he resumed TTFields 6 weeks after this operation.
At the time of writing, the patient remains well and is one of the longest survivors of unmethylated MGMT promoter GBM in Hong Kong.
Neurosurgeon’s perspective
Prior to the emergence of positive data on the use of TTFields beyond progression, due to the absence of efficacious therapies, little consensus existed on second-line GBM treatment, with clinical trials often being recommended as the best option. The US FDA’s approval of TTFields in 2011 was based on a phase III trial comparing the efficacy of TTFields alone vs active chemotherapy in recurrent GBM patients, which failed to show any improvement in OS or a statistically significant improvement in PFS, yet whose QoL and toxicity analyses clearly favoured TTFields.1
Since TTFields therapy does not interact with other systemic therapies, it can be viewed as a local treatment and has few contraindications. For patients receiving craniectomy, which is necessary in very rare cases to alleviate the pressure on the brain, the use of TTFields may interfere with wound healing due to skin irritation. In addition, when setting up the TTFields device for individual patients, neurosurgeons review the preoperative scans to identify the exact position of the tumour and map the precise area of the brain where TTFields should be applied. This mapping model is designed for patients with intact skulls and is based on the distance of the tumour from the skull. Thus, a skull that is no longer intact could potentially interfere with TTFields efficacy. However, craniectomy would only constitute a relative contraindication; the only absolute contraindication would be allergy to the device adhesives.
To assess TTFields’ full potential, each patient’s PS and level of available support should be taken into account. Since the patient’s entire scalp needs to be regularly clipped and the device arrays need to be reapplied every 3 days, the degree of care-giver stress would be considerable.
TTFields improves survival without a negative impact on health-related QoL. Deterioration-free survival is significantly longer with TTFields vs TMZ for global health (4.8 months vs 3.3 months; p<0.01), physical (5.1 months vs 3.7 months; p<0.01) and emotional functioning (5.3 months vs 3.9 months; p<0.01), pain (5.6 months vs 3.6 months; p<0.01), and leg weakness (5.6 months vs 3.9 months; p<0.01), which was likely related to improved PFS.2
QoL directly impacts compliance with TTFields. In the EF-14 trial, compliance of >90 percent (>21.6 hours a day) was associated with a median OS of 24.9 months and an unprecedented 5-year survival rate of 29.3 percent.3 Interestingly, in another subgroup analysis of this trial among South Korean patients, who had particularly high compliance rates, had a median OS that was longer than that in the intention-to-treat population.4,5
Case 2
History and presentation
In August 2019, a 53-year-old female, who enjoyed good past health, presented with an abrupt onset of neurological symptoms, including severe headache lasting several days. While the patient was able to walk unassisted, her KPS was 70.
MRI scan revealed a sizeable (5 cm in the longest dimension), rapidly growing necrotic tumour in the basal ganglia of the thalamus and the left frontal lobe. The depth of the tumour and infiltration into the brain stem made gross total resection impossible, allowing only a biopsy, which also suggested unfavourable prognosis due to wild-type IDH-1 and IDH-2 and unmethylated MGMT promoter.
Treatment and response
The Stupp regimen (radiotherapy, 60 Gy, 30 fractions plus concomitant and adjuvant TMZ) was initiated in August 2019 and completed in November 2019 – 2.5 months after biopsy.6 TTFields was added to ongoing adjuvant TMZ, as per the National Comprehensive Cancer Network’s guidelines, in December 2019.7
The patient experienced considerable neutropenia, thrombocytopenia, intensifying headache, dysphasia, and dysphagia during TMZ therapy. As well as causing speech difficulties, the swelling in the left frontal lobe led to weakness in the right limbs. After 3 months of TMZ treatment, the patient became wheelchair-bound. Her KPS deteriorated to 40 and she was considered for hospice care. On the other hand, she tolerated TTFields well, with scalp abrasion and pruritis as the only AEs, which were controlled by a topical steroid cream.
MRI scans in February and April 2020 revealed continued tumour progression (7 cm in the longest dimension) with further necrotic changes, considerable oedema and increasing intracranial pressure, resulting in a midline shift. The oedema had to be controlled with high-dose steroids, causing the patient to gain almost 20 kg of body weight.
Having excluded pseudoprogression due to early AEs of radiotherapy, the patient’s condition was declared a true progression in April 2020, and a diagnosis of persistent GBM unresponsive to first-line treatment was made. TMZ was stopped, while TTFields was continued.
In an attempt to improve the patient’s QoL, the anti-VEGF therapy bevacizumab was used.8 Bevacizumab in combination with TTFields was very well tolerated. The patient’s limb power, gait, ability to walk, self-care, and dysphasia began to improve within 3 weeks of initiating bevacizumab. Within 4 weeks, her KPS improved to 70. Systemic steroids were stopped and the patient lost 10 kg from her peak body weight, which also aided her ability to walk through improving her balance.
Following six cycles of bevacizumab with continuous TTFields, the patient became well enough for irinotecan to be added to her treatment regimen in June 2020.9 At this time, her tumour had shrunk to 3 cm in the longest dimension.
At the time of writing, the patient remains well, and is able to care for herself and walk unaided.
Discussion
The decision to continue TTFields therapy beyond progression was based on the post hoc analysis of the multicentre, open-label, randomized EF-14 trial, which compared the efficacy of TTFields plus physician’s choice of systemic therapy (n=144) vs systemic therapy alone (n=60) after first GBM recurrence, following first-line treatment with TMZ and TTFields.10 Bevacizumab was the most common systemic therapy used in combination with TTFields, which is reflective of clinical practice for second-line management of GBM patients in Hong Kong. At 12.6 months’ median follow-up, the primary endpoint of median OS was significantly longer in the TTFields plus systemic therapy vs systemic therapy alone (11.8 months vs 9.2 months; hazard ratio [HR], 0.70; 95 percent confidence interval [CI], 0.48 to 1.00; p=0.049).9
While a numerically higher incidence of grade 3/4 treatment-emergent AEs was observed in the TTFields plus systemic therapy (49 percent) vs physician’s choice of systemic therapy alone group (33 percent), this may have been a consequence of the longer duration of follow-up in the TTFields plus systemic therapy group, due to improved OS. Notably, no grade 3/4 seizures were reported in either group, which was the primary concern with TTFields’s tolerability in view of its mode of action.9
Although the use of bevacizumab alone or in combination with chemotherapy was not shown to improve OS, it provided QoL improvements through minimizing steroid use, as in the case of our patient.11,8 While tumour size has a considerable impact on symptoms, intracranial oedema sometimes plays an even greater role. Our patient experienced a remarkable reduction in oedema with bevacizumab in combination with TTFields, suggesting that the two modalities work well together. Furthermore, while the 6-month PFS rates for bevacizumab ± chemotherapy attained in the randomized trials have been between 25–46 percent for patients with recurrent GBM, our patient has enjoyed a sustained benefit from treatment, with her symptoms remaining very stable for nearly 8 months, which is likely due to the combined effect of TTFields and bevacizumab.12,9
Bevacizumab and TTFields have different and independent mechanisms of action. While bevacizumab inhibits tumour angiogenesis, TTFields works by disrupting cancer cell division – as a result, there is no overlapping toxicity.
Due to its mechanism of action, bevacizumab is prone to causing wound healing complications and bleeding, including intracranial haemorrhage.13 However, our patient has tolerated bevacizumab well, and the minor scalp abrasion caused by continuous daily use of TTFields did not impact her QoL or show any bleeding, in spite of bevacizumab use.