
History, presentation and initial treatment
A 60-year-old female complained of dizziness and weakness over the left side of her body (assessed strength, 4/5) when she presented in May 2019. CT scan of the brain revealed a mass measuring approximately 5 cm in the right temporal region and some compression of the ventricles. Craniotomy to excise the suspected malignant brain tumour was carried out in the same month, but was incomplete. The patient’s preoperative Eastern Cooperative Oncology Group (ECOG) performance status (PS) was 1.
Post-excision histopathology confirmed the diagnosis of IDH wild-type, unmethylated MGMT promoter glioblastoma multiforme (GBM). The patient regained full strength of the left side of her body after the surgery.
Adjuvant treatment and response
Radiotherapy, 60 Gy given in 30 fractions over 6 weeks, plus concomitant temozolomide (TMZ) was initiated in July 2019 and completed in August 2019. The patient tolerated the radiochemotherapy well and received the first cycle of adjuvant TMZ chemotherapy in September 2019.
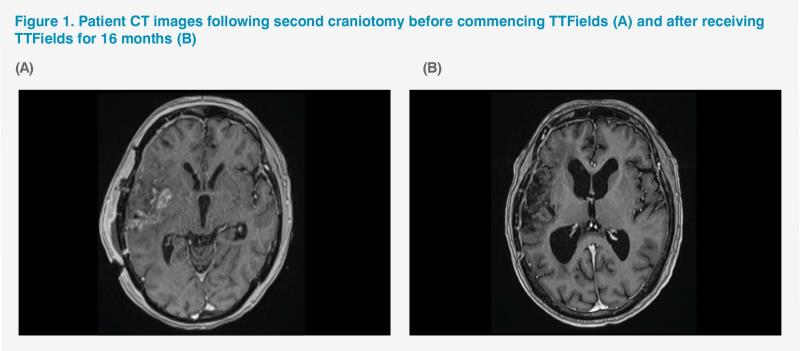
Follow-up CT scan in September 2019 found a 3–4 cm enhancement (exerting less compression effect than the original 5 cm tumour mass) over the previous tumour site. The patient underwent a second craniotomy in October 2019 for gross total resection of the residual tumour. As the region of enhancement was deemed to be incomplete excision rather than progressive disease, adjuvant chemotherapy was resumed. Her second cycle of TMZ started in November 2019 and tumour-treating fields (TTFields) therapy was initiated at the same time. The required six cycles of TMZ were completed in March 2020, while TTFields therapy continued. The patient’s condition remained stable and MRI scan in the same month showed reduced size of the tumour region. (Figure 1A)
Subsequent MRI scans in July and October 2020 and March 2021 showed stable, static disease with no new lesions. (Figure 1B) The patient’s next follow-up MRI scan is due in September 2021. Last seen in the clinic for review in August 2021, the patient came in a wheelchair but reported that she was able to walk with some aid at home. Her ECOG PS remained stable at 1–2. At the time of writing, the patient had been receiving TTFields for 21 months without disease progression and tolerating it well, with some mild dermatitis over the scalp that did not require any topical treatment.
Discussion
Radiotherapy at a dose of 60 Gy given in 2.0 Gy fractions plus concurrent TMZ and adjuvant TMZ (frequently referred to as the Stupp protocol) plus TTFields after surgical resection is given a category 1 treatment recommendation by the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for newly diagnosed GBM patients ≤70 years of age with good PS.1 Our patient responded well to this standard-of-care (SoC) treatment.
TTFields is an antimitotic treatment modality consisting of low-intensity, intermediate-frequency (200 kHz) alternating electric fields delivered via wired transducer arrays placed on the patients’ shaved scalp and connected to a portable device. TTFields cause selective mitotic arrest and apoptosis of rapidly dividing glioblastoma cells.2
TTFields therapy was added to the standard Stupp protocol as a recommended SoC on the basis of results of the open-label phase III EF-14 clinical study involving 695 patients with newly diagnosed, histologically confirmed GBM and Karnofsky PS ≥70, who had undergone debulking surgery or biopsy and had completed standard radiotherapy with concomitant TMZ at the time of enrolment. Patients were randomized 2:1 to receive TTFields plus maintenance TMZ or TMZ alone. The primary endpoint was progression-free survival (PFS), while the secondary endpoint was overall survival (OS).1,2
After a median follow-up of 40 months and a minimum follow-up of 24 months, median PFS was 6.7 months for patients treated with TTFields plus TMZ vs 4.0 months in patients treated with TMZ alone (hazard ratio [HR], 0.63; 95 percent confidence interval [CI], 0.52 to 0.76; p<0.001). Notably, our patient remains progression-free 21 months after initiating TTFields therapy.
Median OS was 20.9 months in the TTFields plus TMZ group vs 16.0 months in the TMZ-only group (HR, 0.63; 95 percent CI, 0.53 to 0.76; p<0.001).2
A secondary analysis of the EF-14 trial assessing the influence of TTFields on health-related quality of life (HRQoL) showed that the addition of TTFields to TMZ improved survival without a negative impact on HRQoL, except for scalp itchiness.3 Deterioration-free survival was significantly longer with TTFields plus TMZ vs TMZ alone for global health (4.8 months vs 3.3 months; p<0.01), physical (5.1 months vs 3.7 months; p<0.01) and emotional functioning (5.3 months vs 3.9 months; p<0.01), pain (5.6 months vs 3.6 months; p<0.01), and leg weakness (5.6 months vs 3.9 months; p<0.01). Time to deterioration, reflecting the influence of treatment, did not differ significantly between groups, except for itchy skin (worse with TTFields; 8.2 months vs 14.4 months; p<0.001) and pain (better with TTFields; 13.4 months vs 12.1 months; p<0.01). Role, social, and physical functioning were not affected by TTFields.3
QoL directly impacts compliance with TTFields.3,4 Effective treatment with TTFields requires good patient compliance and support, as the patient has to carry the mobile electrical device for at least 18 hours/day with four arrays of transducers continuously fixed to the scalp.2,4 A post-hoc analysis of the EF-14 trial showed that compliance rates >90 percent were associated with extended median survival (24.9 months vs 20.9 months overall) and a 5-year survival rate of 29.3 percent.2,4
Despite the effectiveness and favourable HRQoL data of TTFields demonstrated, some patients may decline this treatment modality as they find it inconvenient or do not have a supportive caregiver. Our patient’s husband was able to provide constant care, as well as the psychological and practical support she needed, including helping her with the challenging task of reattaching the electrodes onto her scalp after her shower and daily break from treatment, enabling her to achieve compliance of 22 hours/day (>90 percent).
Other than the minor complaint of having to carry the device (1.2 kg), our patient tolerated TTFields well, with some skin dryness and itchiness at the site of contact with the array being the only side effect, which did not require any topical treatment.5 Interestingly, a subgroup analysis of the EF-14 trial found that skin irritation was reported by a smaller proportion of Asian (Korean) patients vs the overall population (30 percent vs 52 percent).2,6