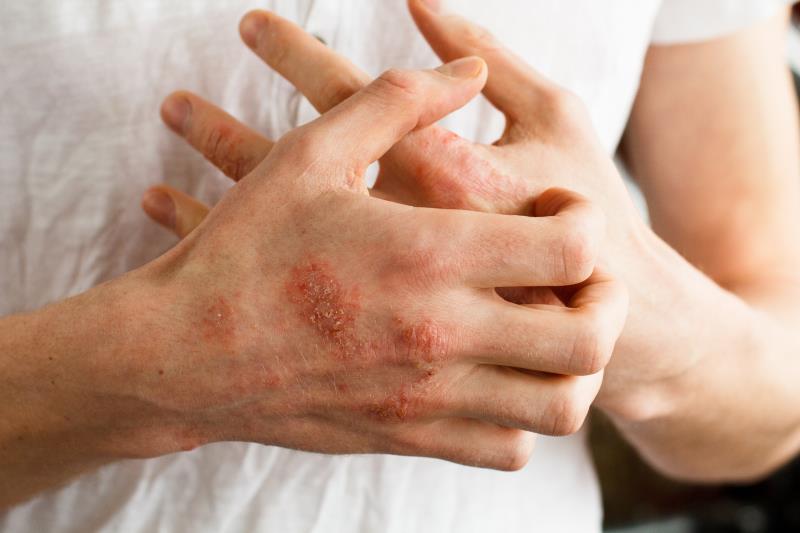
Treatment with baricitinib led to clinically meaningful improvements in itch and skin symptoms in patients with moderate-to-severe atopic dermatitis (AD), even in those with additional comorbid atopic conditions, according to a pooled analysis of two phase III studies released in the AAAAI 2020 Meeting.
“Asthma and allergic rhinitis are frequent atopic comorbidities in AD and may influence the management of patients with AD,” said the researchers.
“[In the current pooled analysis of studies on baricitinib,] there were no treatment-effect differences between groups of patients with or without comorbidity,” they added.
Baricitinib is a selective and reversible oral inhibitor of Janus kinase (JAK) 1 and JAK2, which has previously been approved for treatment of rheumatoid arthritis.
The current analysis pooled data from two phase III, double-blind trials of baricitinib in patients with moderate-to-severe AD: BREEZE-AD1 (n=624) and BREEZE-AD2 (n=615). Patients were randomized in a 2:1:1:1 ratio to receive placebo or baricitinib 1 mg, 2 mg, or 4 mg once daily for 16 weeks. [AAAAI 2020, abstract 610]
As high as 71 percent (n=878) of the patients had at least one atopic comorbidity, with allergic rhinitis being the most common (57.7 percent), followed by seasonal allergy (45.9 percent), asthma (41.2 percent), and food allergy (40.7 percent).
Among patients with atopic comorbidities, there were significantly more patients who attained an IGA* response (IGA≤2; disease rated as mild or less) in all the baricitinib groups compared with placebo at week 16 (29.8 percent, 25.0 percent, and 18.6 percent vs 9.8 percent for 4-, 2-, and 1-mg baricitinib vs placebo, respectively; p≤0.01 for all).
Similar improvements were seen in the proportion of patients achieving ≥50 percent reduction in EASI** score (EASI50), in favour of baricitinib over placebo (32.7 percent, 29.0 percent, and 20.3 percent, vs 11.5 percent; p≤0.01 for all).
Improvements in itch symptoms were also greater with baricitinib than placebo among the patients with atopic comorbidities, as indicated by ≥4 points improvement on the Itch Numeric Rating Scale (21.6 percent, 16.6 percent, and 10.1 percent vs 4.4 percent; p≤0.05 for all).
In addition, baritinib consistently improved sleep disturbance due to itch at week 16 among the patients with comorbid atopic conditions. According to the researchers, moderate-to-severe AD is characterized by severe itching, which can lead to damaged skin and sleep loss.
When comparing the treatment effects in patients with atopic comorbidities vs those without, the benefits of baricitinib were consistent for all outcomes mentioned above.
“Overall safety outcomes for patients with atopic comorbidities were similar to outcomes in the overall trial population,” the researchers reported.
Recently released data from the BREEZE-AD5 trial showed that the 2-mg dose of baricitinib monotherapy met its primary endpoint of ≥75 percent improvement in EASI at week 16 compared with placebo.
According to the principal investigator, the results support the potential of baricitinib as an additional treatment option for patients with moderate-to-severe AD, in whom treatment choices are otherwise limited.