Roflumilast cream may reduce severity of plaque psoriasis




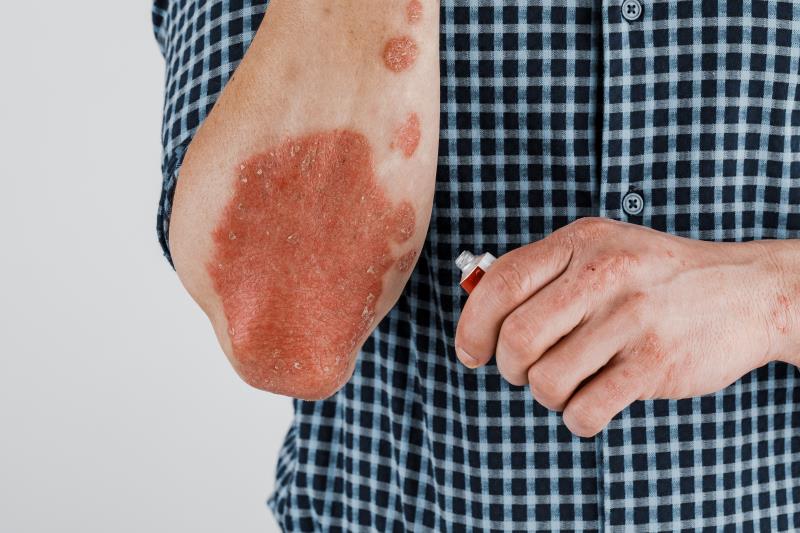
Use of the topical PDE-4* inhibitor roflumilast led to greater reductions in the signs and symptoms of chronic plaque psoriasis compared with placebo, a phase IIb trial has shown.
“[W]e found that roflumilast cream was efficacious in reducing the severity of psoriasis according to an investigator assessment of a state of clear or almost clear of plaque psoriasis (IGA** score of 0 or 1, respectively),” said the researchers.
At 6 weeks, more roflumilast recipients exhibited an IGA score of 0 or 1 vs those on placebo (28 percent vs 8 percent; p<0.001 [0.3% cream] and 23 percent vs 8 percent; p=0.004 [0.15% cream]). These rates increased by week 8 (38 percent [0.3%], 31 percent [0.15%], and 12 percent [placebo]) and week 12 (38, 32, and 16 percent, respectively). [N Engl J Med 2020;383:229-239]
“[Although the] outcomes with the higher concentration of roflumilast … were numerically better than those with the lower dose … no formal comparisons were made between dose levels,” the researchers noted.
Intervention-related adverse events (AEs) were observed in 6 percent and 3 percent of roflumilast 0.3% and 0.15% recipients, respectively. Fewer application-site reactions were noted with the 0.15% vs the 0.3% roflumilast cream (n=1 vs 4).
Sensitive areas included
Adults with mild, moderate, or severe chronic plaque psoriasis*** were included in the analysis. A total of 331 participants were randomized 1:1:1 to apply roflumilast 0.3% or 0.15% cream, or a vehicle placebo cream, once daily to all lesions – including sensitive (ie, facial and intertriginous) areas – for 12 weeks.
Among those with mild intertriginous psoriasis (~15 percent), more roflumilast vs placebo recipients achieved an IGA score of 0 or 1, plus a 2-grade improvement in the intertriginous area IGA score (73 percent [0.3%] and 44 percent [0.15%] vs 29 percent [placebo]). By week 12, almost all roflumilast 0.3% recipients in this subgroup (n=14/15) achieved an intertriginous area IGA score of 0 vs three out of 17 placebo recipients.
“Treatment of sensitive areas … with topical agents requires special consideration because of the sensitivity of the skin in these areas as well its thinness – factors that potentially enhance systemic absorption,” said the researchers.
Choosing the PDE-4 route
While there are effective topical glucocorticoids for psoriasis, their use is confined to no more than 2–8 weeks, and is associated with gradually resolving or irreversible AEs. [Am Fam Physician 2009;79:135-140; Indian Dermatol Online J 2014;5:416-425] Topical calcineurin inhibitors, which have been used off-label for facial and intertriginous psoriasis, are ineffective in nonfacial sites and has a cancer risk potential. [Arch Dermatol 2005;141:1154]
PDE-4 inhibition downregulates immune modulators#. [Front Pharmacol 2018;9:1048] However, the approved oral PDE-4 inhibitor apremilast is confounded by gastrointestinal (GI) side effects (eg, diarrhoea, nausea), noted the researchers. “[Our findings show that] topical administration of roflumilast cream was associated with an incidence of <1 percent of [GI] events … possibly as a result of bypassing the [GI] tract with topical administration,” they said.
Overall, the findings provide evidence of a topical PDE-4 inhibitor alternative for the treatment of psoriasis, for which no topical PDE-4 inhibitor is approved, noted the researchers. Larger and longer trials are thus warranted to ascertain the safety and durability of response of roflumilast in this setting.