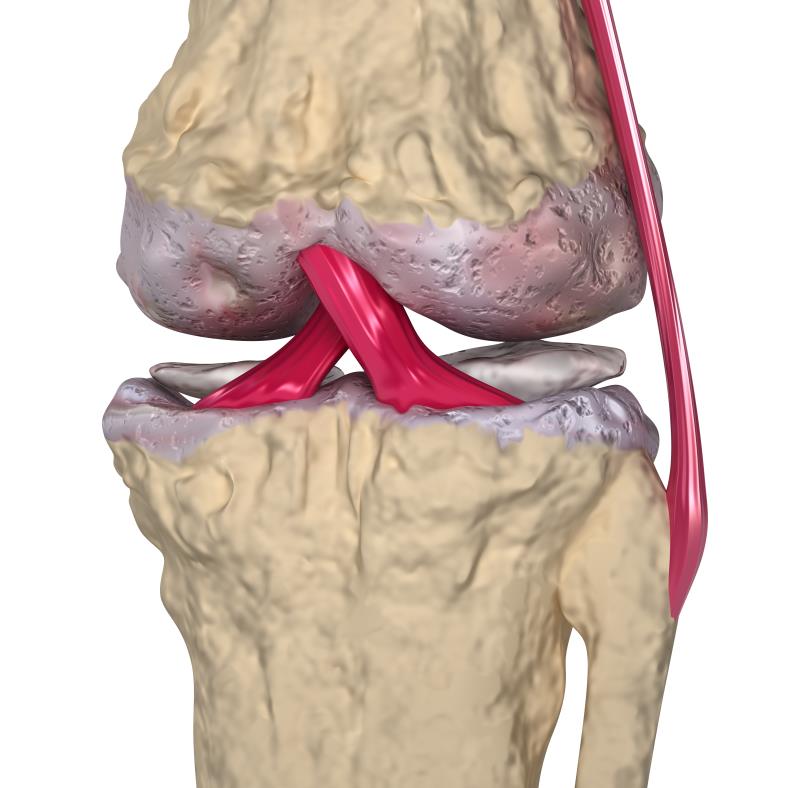
Annual infusions of zoledronic acid did not significantly cut pain and cartilage volume loss in patients with symptomatic knee osteoarthritis (OA) and bone marrow lesions, the ZAP2* trial has shown.
Given the resultant disability, impaired quality of life, and economic burden of knee OA, alleviating pain and preventing structural progression are two major treatment goals. [Nat Rev Dis Primers 2016;2:16072] “However, pain control remains poor in more than half of patients, and no approved disease-modifying therapies have been identified that prevent structural progression of knee OA,” said the researchers.
“[Our] findings do not support the use of zoledronic acid for slowing cartilage volume loss or alleviating knee pain in [this setting],” they said.
At 2 years, mean reductions in tibiofemoral cartilage volume were not significantly different between zoledronic acid and placebo (–878 mm3 vs –919 mm3; p=0.5). [JAMA 2020;323:1456-1466]
There were also no remarkable changes between zoledronic acid and placebo in terms of pain parameters (–11.5 vs –16.8; p=0.17 [VAS**] and –37.5 vs –58.0; p=0.21 [WOMAC***]) and bone marrow lesion size (–33 mm2 vs –6 mm2; p=0.6). Although these results differed from a pilot study using a similar protocol, the findings in the latter could have been “due to chance”, noted the researchers, as these were not replicated in ZAP2. [Ann Rheum Dis 2012;71:1322-1328]
Adverse events, which were more frequent with zoledronic acid than placebo (96 percent vs 83 percent), were primarily acute reactions (eg, musculoskeletal pain and stiffness, fever, headache, and dizziness). These gradually dissipated following the second infusion.
The study comprised 223 participants (mean age 62 years, 52 percent female) who were randomized 1:1 to receive single infusions of zoledronic acid 5 mg (in 100-mL saline) or a placebo saline solution at baseline and at 12 months. Of these, 85 percent completed the trial.
While the planned sample size was not reached, it is unlikely that a clinically important effect was missed for any of the outcomes, noted the researchers. Moreover, despite the misadministration of zoledronic acid in one patient assigned to placebo, per-protocol analyses generated similar results. This suggests no effect from misallocation, they added.
The loss to follow-up rate was higher with zoledronic acid vs placebo (20 percent vs 9 percent), which as per the researchers, was primarily due to the higher knee replacement rates with the former than the latter (9 percent vs 2 percent). “This [difference] has the potential to influence the missing at random assumption used for addressing missing data.”
Nonetheless, the study supports evidence reflecting the lack of efficacy of bisphosphonates on preventing the progression of knee OA, [Arthritis Res Ther 2005;7:R625-R633; Arthritis Rheum 2006;54:3494-3507] with several factors accounting for the strength of the findings.
ZAP2 used the most potent IV bisphosphonate, and the annual administrations contributed to increased adherence rates, said the researchers. “[ZAP2 had] patients with a subchondral bone marrow lesion who may be more likely to benefit from bisphosphonate therapy,” they added. Moreover, the exclusion of patients with severe radiographic JSN#, who only have a small volume of cartilage remaining, minimized a floor effect.
Other reinforcing factors are the longer follow-up which offered ample time to observe alterations in radiographic JSN, and the utilization of MRI. “[MRI] is more sensitive to change compared with a surrogate measure of cartilage volume (ie, radiographic JSN),” said the researchers.