Cetirizine an attractive alternative to diphenhydramine for hives




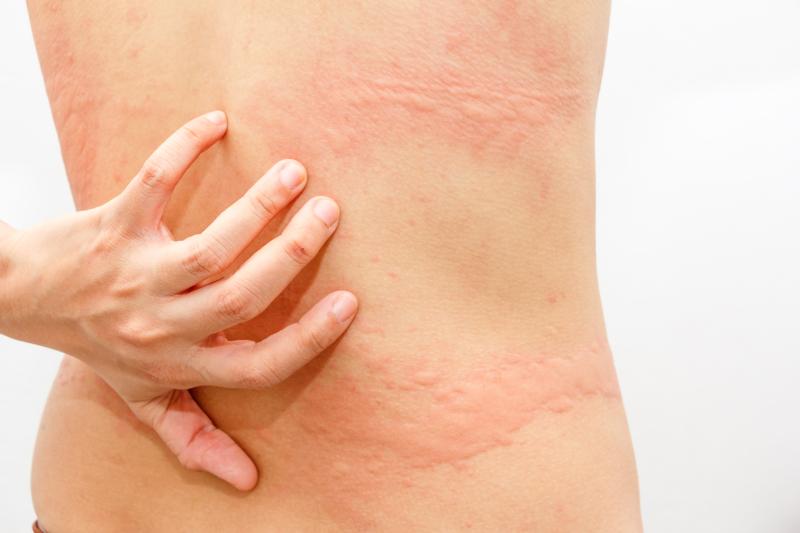
Cetirizine drip for treating acute urticaria is as effective as intravenous (IV) diphenhydramine, with the added benefits of less sedation, fewer adverse events, shorter treatment time, and lower rates of revisit to treatment centre, according to the results of a phase III study.
“Acute urticaria typically responds to pharmacotherapy, with antihistamines being the first-line treatment. The parenteral route of administration is often preferred to provide rapid onset of action,” the investigators said. [J Allergy Clin Immunol 2014;133:1270-1277; Am Fam Physician 2017;95:717-724]
IV diphenhydramine has been a treatment standard, but like other first-generation antihistamines, the drug is known to have sedating effects that can cause significant driving impairment comparable to or greater than that of alcohol intoxication (blood alcohol level of 0.1 percent), leading to recommendations that patients not drive after treatment, they pointed out. [Hum Psychopharmacol 2016;31:167-177; Ann Allergy Asthma Immunol 2004;92:294-303]
In the current trial, 262 acute urticaria patients (mean age, 39 years; 63 percent female) who presented to emergency departments (EDs) and urgent care centres requiring an IV treatment were randomized to receive either the second-generation antihistamine cetirizine 10 mg (n=127) or the first-generation diphenhydramine 50 mg (n=135).
Cetirizine-treated patients experienced less sedation than those who received diphenhydramine. The corresponding mean patient-rated sedation scores (from 0 indicating not drowsy at all to 3 being extremely drowsy) were 0.2 vs 0.7 (p=0.003) in the first hour following treatment, 0.1 vs 0.5 (p=0.03) at the 2-hour assessment, and 0.1 vs 0.5 (p=0.04) at discharge. The same pattern of changes was observed among older patients (age ≥65 years). [Ann Emerg Med 2020;doi:10.1016/j.annemergmed.2020.05.025]
Cetirizine was also associated with fewer adverse events (AEs; 3.9 percent vs 13.3 percent). Of the 31 AEs, only seven occurred with the drug. Dizziness and nausea were the most common AEs, and all were documented in the diphenhydramine arm (4.4 percent and 3.0 percent, respectively).
“The safety and tolerability of cetirizine have been well established during the past 30 years,” the investigators noted, adding that its IV and oral formulations have similar safety profiles. [Drugs 2004;64:523-561]
In terms of efficacy, the 2-hour pruritus score change from baseline with cetirizine was noninferior to that with diphenhydramine (–1.6 vs –1.5, respectively, 95 percent confidence interval, –0.1 to 0.3; p=0.35). This held true in subgroups of patients defined by age (<65 and ≥65 years).
The number of “effectively treated” patients according to physician assessment was higher in the cetirizine arm (p=0.02), and more patients required rescue drug use in the diphenhydramine arm (p=0.016). The most common rescue medication was corticosteroids in both groups.
Cetirizine was also associated with shorter time spent in treatment centre (1.7 vs 2.1 hours; p=0.005) and fewer instances of returning to the centre (5.5 percent vs 14.1 percent; p=0.02).
“Overall, these clinical trial data have applicability for emergency medicine as well as other settings, including emerging models of care in which safety and sedation will be of paramount importance,” the investigators said.
“Although economic outcomes were not captured in the current clinical trial, the observed reductions [in] time spent in ED, ED readmission rate, and rescue medication use with IV cetirizine may confer cost savings,” they added.