Tafulprost is a safe and effective treatment for lowering intraocular pressure (IOP) in glaucoma patients, a recent Philippine study has shown.
Researchers retrospectively reviewed 329 eyes of 177 glaucoma patients (mean age, 64.8 years) who were on 0.0015% tafulprost medication. Patients were followed for at least 3 months. The main outcome was the mean change in IOP after 3 months. Secondary outcomes included adverse events and longitudinal IOP measurements.
IOP in the entire cohort dropped by a mean of 6.18±4.06 mm Hg over 3 months of follow-up. This corresponded to a 26.37-percent decrease from the baseline value of 23.44 mm Hg (p<0.001). This drop was retained until month 12 (p<0.001).
Stratified analysis did not change the principal findings. Among patients who were treatment-naïve at baseline, IOP dropped by 8.34 mm Hg by month 3 from a baseline figure of 26.70 mm Hg. The 31.24-percent decrease was statistically significant. Similarly, among those who received tafulpost as a replacement therapy, IOP declined by 1.00±3.08 mm Hg (–6.31 percent of 15.86 mm Hg; p=0.007).
Fifty-three patients were given tafulprost as an add-on therapy. In these patients, IOP decreased by 5.08±2.86 mm Hg from baseline (–23.68 percent of 21.45 mm Hg; p<0.001).
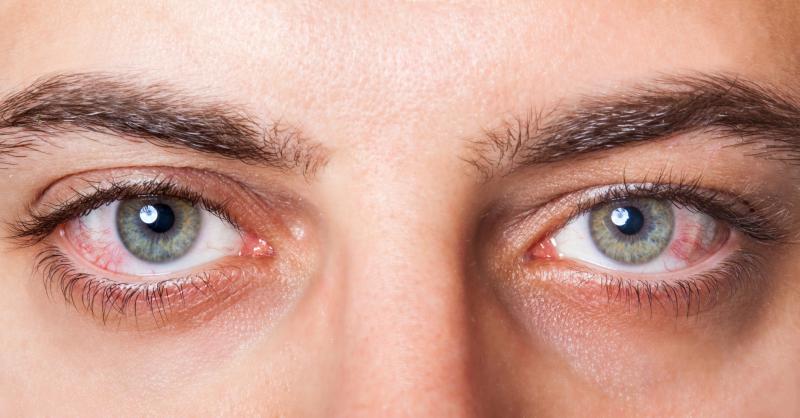
These clinical benefits did not come with adverse events for at least 85 percent of the patients. Conjunctival hyperaemia was reported by 15 percent of the sample and was the most common adverse event. Eye redness was the most frequent symptom of side effects. There were no treatment discontinuations.