 Dr Michael J. Alexander (AHA photo)
Dr Michael J. Alexander (AHA photo)Stenting cholesterol-blocked arteries in the brain appears to reduce recurrent stroke risk in patients with intracranial atherosclerotic disease (ICAD), one-year results of the WOVEN* trial, presented at the International Stroke Conference (ISC) 2020, have shown.
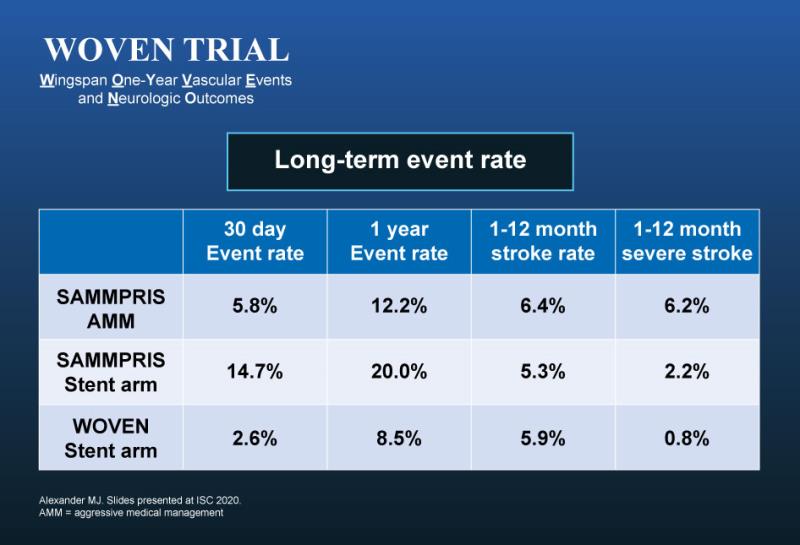
Stroke or death rate at 1 year was 8.5 percent in 129 patients stented with Stryker’s Wingspan Stent System (Wingspan) and that included four patients who had periprocedural events, reported WOVEN trial investigator Dr Michael J. Alexander, professor and vice chairman of neurosurgery at Cedars-Sinai Medical Center in Los Angeles, California, US. [ISC 2020, abstract LB4]
The stroke rate in WOVEN was comparable to the stenting arm of the SAMMPRIS** trial, which compared medical therapy and angioplasty vs stenting in stroke patients. [N Engl J Med 2011;365:993-1003]
“But because the rate of periprocedural complication was very low in the WOVEN trial at 30 days (2.6 percent), the overall 1-year stroke and death rate of 8.5 percent trended lower vs aggressive medical therapy alone in SAMMPRIS,” Alexander emphasized.
The restenosis rate was 16.8 percent in this cohort, and 7 of 18 patients (38.9 percent) were symptomatic, he said. “This was the primary cause of delayed stroke within the first year post-stenting.”
Largest on-label intracranial stenting trial to date
The WOVEN trial, conducted at 16 US centres, was a physician-initiated follow-up of the FDA-mandated WEAVE*** trial, which sought to assess the periprocedural safety of the Wingspan stent for the treatment of symptomatic ICAD.
The WEAVE trial was stopped early after an interim analysis of the 152 stented patients (who met the FDA-approved indications for use) showed a lower than expected 2.6 percent (4 of 152 patients) periprocedural stroke, bleed, and death rate in stented patients, lower than the 4 percent periprocedural primary event safety benchmark set for the interim analysis. At 72 hours post-stenting, 97.4 percent (n=148) of the patients were event-free, 1.3 percent (n=2) had nonfatal strokes, and 1.3 percent (n=2) had died. A significantly higher incidence of stroke or death occurred within 72 hours of the procedure when the Wingspan was used outside of the FDA-approved indications for use. [Stroke 2019;50:889-894]
Patients stented with the Wingspan stent in the WEAVE trial were followed-up for a year in the WOVEN trial. Subsequent strokes and deaths were analysed, so was restenosis rate. [ISC 2020, abstract LB4]
 The Wingspan stent (image courtesy of Stryker)
The Wingspan stent (image courtesy of Stryker)“WOVEN is unique because prior studies of the stent included off-label patients,” commented Alexander. “This is the largest intracranial stent trial for atherosclerotic disease performed according to the US FDA indication for the Wingspan stent,” he said.
“It is interesting to note that the stroke and death rates were substantially lower than the one-year rate of 20 percent in the stenting arm of the SAMMPRIS trial and slightly better than the 12.2 percent stroke and death rate in the medical arm of SAMMPRIS.”
Stenting patients who failed medical therapy
“The stroke community is well aware of the results of the SAMMPRIS trial that tested stenting vs best medical therapy … [but] the study did not show any benefit in these patients,” commented Dr Mitchell S.V. Elkind from the Columbia University in New York City, New York, US and president-elect, American Heart Association. [https://newsroom.heart.org/news/follow-up-study-suggests-brain-stents-are-safe-and-effective-for-reducing-recurrent-stroke-risk?preview=3d6cf4f20305d2e41d4bb26351c91ed9]
There’s always been this residual question of whether there is a benefit to stenting this subgroup of patients with intracranial stenosis who failed medical therapy, and who despite all the best drugs and things thrown at them, continue to have symptoms, he added.
Atherosclerotic intracranial arterial stenosis is one of the most common causes of stroke and is associated with a high risk of recurrent stroke. [Circulation 2006;113:555-563; Stroke 2003;34:2361-2366]
“The WOVEN trial looked at 150 patients who had stents placed in intracranial vessels according to the FDA indication for using the Wingspan stent. There was a low risk of recurrent stroke or death in these patients at 1 year, compared to what we would expect historically. About 9 percent of the patients had a recurrent event vs what we would expect to be closer to about 20 percent. This is a registry, not a head-to-head comparison, nor a randomized trial. So, we’re a bit limited as to the conclusions we can definitively draw from it. But results are promising and suggest that for some patients, the benefits of stenting may be better than what we would expect if we treat them only historically.”
Experts’ perspective
Other experts said the results of the WOVEN trial offer greater confidence in using the Wingspan stent in patients who best fit the criteria for its use.
“The data [from WOVEN] are reassuring in terms of the recurrent stroke and restenosis rates,” commented Professor Yu Chun Ho Simon from the Department of Imaging and Interventional Radiology, The Chinese University of Hong Kong, Hong Kong, China. “Our study of the Wingspan stent in Hong Kong yielded even better results as recurrent ipsilateral ischaemic stroke rate was zero and in-stent restenosis was 13.6 percent at 1-year post stenting.” [J NeuroIntervent Surg 2014;6:96–102]
 Prof Yu Chun Ho Simon
Prof Yu Chun Ho SimonMeanwhile, Professor Jung Hwa Seo from the Department of Neurology, Busan Paik Hospital, Inje University, Busan, Korea, who is not involved in the WOVEN study, said: “My first-line treatment for ICAD is aggressive medical management. But the one-year result of the WOVEN trial gave me a better belief that if aggressive medical management for ICAD fails, an angioplasty using the Wingspan stent would help to better manage patients.”
 Prof Jung Hwa Seo
Prof Jung Hwa SeoFuture directions
Stryker’s Wingspan stent is FDA-approved to open narrowed brain arteries in patients 22-80 years old with ICAD who had 2 or more repeated strokes despite aggressive medical management.
Alexander said the long-term results of the WOVEN study are important to determine if safer stenting practices and lower complication rates from the treatment itself resulted in improved patient outcomes at 1 year.
“Intracranial stenting could provide an alternative when medical therapy and other treatments have been unsuccessful.” The WOVEN trial results, he added, strongly support further randomized clinical trials between intracranial stenting and medical therapy for high-risk patients with ICAD and haemodynamic compromise treated in an on-label fashion.