Invasive fungal infections (IFIs), including aspergillosis and mucormycosis, are life-threatening and cause significant morbidity and mortality in patients. At a recent symposium, A/Prof Tan Ban Hock, Senior Consultant at the Department of Infectious Diseases, Singapore General Hospital (SGH), A/Prof Tan Thuan Tong, Senior Consultant and Head of Department, Infectious Diseases, SGH, and A/Prof Andrea Kwa, Clinician Scientist, Pharmacy, SGH, discussed the diagnosis and management of IFIs with a focus on isavuconazole (Cresemba®, Pfizer), a second-generation triazole indicated for the treatment of invasive aspergillosis (IA) and mucormycosis in adult patients for whom amphotericin B is inappropriate. Dr Yvonne Loh, Consultant Hematologist at Raffles Cancer Centre, chaired the event.
Challenges with diagnosis and management
IFIs frequently occur in severely immunocompromised patients. IA is the most common IFI, followed by mucormycosis. Prolonged neutropenia is the main risk factor for IFI, with IA incidences reaching up to 25 percent in this group. [Clin Microbiol Infect 2014;20(Suppl 6):1-4]
The diagnosis of IFI is challenging and often missed. Though there are reference standards for diagnosis, obtaining tissue specimens for culture and histological examination may not always be feasible. Hence, clinicians often rely on a combination of less-specific clinical, laboratory, and radiological data, and situations that present significant diagnostic uncertainty are the norm. Differentiating IA from mucormycosis is also difficult. [Clin Microbiol Rev 2014;27:490-526]
In addition, there are currently limited treatment options for IFI, with many being associated with tolerability issues, drug–drug interactions, lack of evidence for use, and require therapeutic drug monitoring (TDM). [Ann NY Acad Sci 2012;1272:23-30]
Delayed treatment increases mortality. For IA, overall mortality rates range from 41 to 60 percent. [Clin Microbiol Infect 2011;17:1882-1889] Likewise, outcomes for mucormycosis are poor, with overall mortality rates ranging from 44 to 65 percent, and up to 95 percent, if untreated. [Clin Microbiol Infect 2014;20(Suppl 6):67-73]
The risk of IA may be stratified as low, intermediate, and high risk, based on primary host factors, said Dr Tan Thuan Tong. “A prophylactic approach to IFI is appropriate in high-risk patients; however, a diagnostic-driven treatment approach to other patient groups may be clinically and economically beneficial.”
Diagnosing IF
The diagnosis of IA should be based on the integration of clinical, radiological, and microbiological data. The European Organization for Research and Treatment of Cancer (EORTC) Mycoses Study Group (MSG) guidelines uses only radiological criteria for the clinical component of IA diagnosis. [Clin Infect Dis 2008;46:1813-1821] According to the 2017 European Society for Clinical Microbiology and Infectious Diseases, the European Confederation of Medical Mycology, and the European Respiratory Society (ESCMID-ECMM-ERS) Joint Clinical Guidelines, a multislice, spiral CT of the chest is the imaging modality of choice in patients at risk for IA with fever of unknown origin, or clinical symptoms of lower respiratory tract infection who remain febrile despite broad-spectrum antibacterial treatment. Currently, there is no CT scanning technique that is 100 percent sensitive or specific for pulmonary IA. [Clin Microbiol Infect 2018;24:e1-e38]
Classical CT findings of IA include macronodule(s) >1 cm, which may be surrounded by a halo sign (early phase, inconstant), pleural based wedge-shaped areas of consolidation, alveolar consolidations, masses, internal low attenuation, reversed halo sign, cavity or air-crescent sign (delayed finding), ground glass opacities, and pleural effusion. [Clin Microbiol Infect 2018;24:e1-e38] Interestingly, the clinical and radiological features of IA differ among solid transplant recipients and neutropenic patients. [Transpl Infect Dis 2010;12:309-315]
Reversed halo sign is an early indicator of mucormycosis in the setting of neutropenic leukaemia with pulmonary infection. [Clin Infect Dis 2014;58(5):672-678; Clin Infect Dis 2008;46:1733-1737] However, a firm diagnosis often requires biopsy of the lesion for microscopic, culture, and/or histopathological examination. [Haematologica 2017;102:433-444]
Galactomannan (GM) tests, though widely used for the early diagnosis of IA, is only moderately sensitive. [Clin Infect Dis 2006;42:1417-1427; J Clin Microbiol 2006;44:389-394] Combining GM with polymerase chain reaction (PCR) tests improves the positive predictive value. [Clin Infect Dis 2015;61:1263-1272]
“CT is an important pillar of diagnostic efforts, especially in neutropenic patients. The halo sign is caused by angio-invasion and tissue infarction, with surrounding haemorrhage; it is transient, but is associated with improved prognosis. Reversed halo sign is a clue to mucormycosis. Galactomannan assays are useful for the diagnosis of IA, but accuracy is modest. Combining GM with PCR may work better,” Dr Tan Ban Hock summarized.
Isavuconazole efficacy supported by robust data
The introduction of broad-spectrum antifungal agents and lipid-based formulations of amphotericin B has substantially improved the survival rates in patients with IA and mucormycosis. However, these agents have unpredictable pharmacokinetic profiles and require therapeutic drug monitoring. Isavuconazole is a new potent second-generation triazole which has shown efficacy in the treatment of IA and mucormycosis. It has a favourable pharmacokinetic and safety profile, which may simplify treatment through reduced drug-drug interactions.
In the phase III randomized controlled SECURE trial of 527 patients with suspected invasive mould disease (caused by Aspergillus species or other filamentous fungi), isavuconazole was noninferior to voriconazole for the primary treatment of suspected invasive mould disease. All-cause mortality from first dose of study drug to day 42 in the intent-to-treat (ITT) population was 19 percent with isavuconazole (48 patients) and 20 percent with voriconazole (52 patients), for an adjusted treatment difference of -1.0 percent. This trend persisted to day 84 (Figure 1). [Lancet 2016;387:760-769]
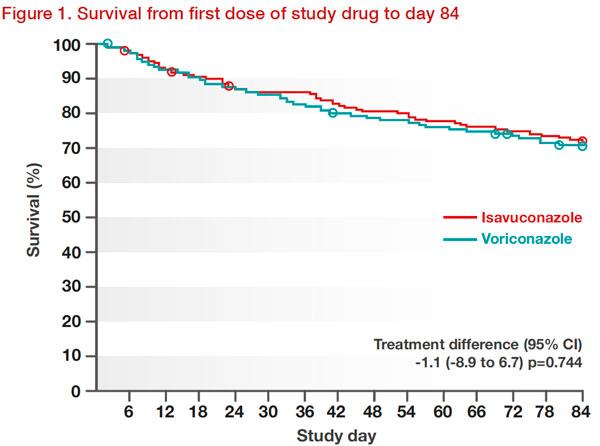
Importantly, isavuconazole was well tolerated than voriconazole, with significantly fewer drug-related adverse events leading to discontinuation (14 percent vs 23 percent) and fewer adverse events of the eye (p=0.002), skin or subcutaneous tissue (p=0.037), and hepatobiliary systems (p=0.016). [Lancet 2016;387:760-769]
Subsequently, major guidelines including the ECIL-6 and ESCMID-EC-MM-ERS, recommend the use of isavuconazole for the first-line treatment of IA. [Haematologica 2017;102:433-444; Clin Microbiol Infect 2018;24:e1-e38]. However, in the IDSA guidelines, isavuconazole remains an alternative therapy and not a primary choice for IA. [Clin Infect Dis 2016;63:e1-e60]
The efficacy and safety of isavuconazole for the treatment of mucormycosis, either for primary treatment, refractory disease, or as an alternative in patients intolerant to amphotericin B, was demonstrated in the VITAL study. By day 42, 11 percent had a partial response to isavuconazole, 43 percent had stable fungal disease, and 3 percent had invasive fungal disease. Isavuconazole showed similar efficacy to that of amphotericin B in case‐matched controls from the Fungi Scope Registry. Weighted all-cause mortality through day 42 was similar for isavuconazole and amphotericin B matched controls (33 percent vs 41 percent; p=0.595). The survival probability through day 84 was similar between groups (Figure 2). [Lancet Infect Dis 2016;16:828-837]
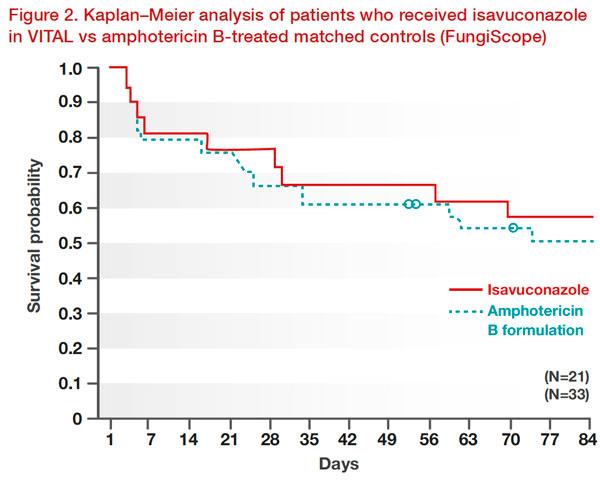
Notably, isavuconazole was well tolerated and toxic effects were an uncommon cause for treatment discontinuation in this patient group. [Lancet Infect Dis 2016;16:828-837]
A post hoc analysis of 15 patients with IFI caused by multiple fungal species, who were originally enrolled in the VITAL study, showed that overall treatment success was 13 percent at days 42 and 84; all-cause mortality was 13 percent and 27 percent at days 42 and 84, respectively. The analysis concluded that isavuconazole may be useful for the treatment of some IFIs caused by multiple fungal species. [Mycoses 2018;61:485-497]
Isavuconazole: pharmacotherapy and drug-drug interactions
Isavuconazole (Cresemba®) is available in both oral and IV formulations. Unlike the other second-generation triazoles, isavuconazole is highly water soluble and does not require the addition of a beta-cyclodextrin to its IV formulation to facilitate solubility, eliminating the potential for nephrotoxicity from the cyclodextrin vehicle. It has a high oral bioavailability of 98 percent, with linear pharmacokinetics of up to 600 mg. There is no clinically significant food effect, nor pH effect. Isavuconazole is metabolized by CYP3A4/5 and UGT. It has a long terminal elimination half-life of approximately 130 hours and requires a loading dose regimen to rapidly achieve steady state. No dose adjustment is required in individuals with renal impairment, including those on dialysis or with mild-to-moderate hepatic impairment. [Antimicrob Agents Chemother 2017;61:e00101-e00117; Drug Des Devel Ther 2018;12:1033-1044]
“All azoles cause drug–drug interactions, and azoles are most often the perpetrator and less often the victim drug,” said Dr Andrea Kwa. “In AML, for example, 80 percent of patients receive concomitant medication that interacts with older triazoles. Azoles differ in inhibitory potential and inhibition is concentration-dependent. Specifically, new emerging drugs such as idelalisib, venetoclax, enasidenib, and ivosidenib pose a challenge to optimal management, and this also goes well for anti-infective drugs such as letermovir.”
Isavuconazole is a moderate inhibitor of CYP3A4, unlike voriconazole and posaconazole which are strong inhibitors, thus isavuconazole may be a better option for use with other new haematological drugs, said Kwa. “However, healthcare professionals should be aware that despite all efforts, there are still unresolved pharmacokinetic drug–drug interactions.”
Is routine TDM required for isavuconazole?
Current guidance strongly recommends TDM for all patients receiving voriconazole treatment or prophylaxis for IA, and in those receiving posaconazole for treatment of IA. As for isavuconazole, there is limited data to support routine TDM, though it may be indicated in the setting of treatment failure and drug interactions, or if toxicity is suspected.
“In AML, over 80 percent of patients receive concomitant medication that prolongs QTc and/ or cause Torsades de Pointes,” added Kwa. While azoles as a class effect are known to prolong QTc interval, clinical trials have shown that isavuconazole administration may cause QTc shortening in a dose-related manner. [Mycoses 2018;61:256-260] This property may be beneficial when used with drugs that prolong QTc.
Key takeaways
The diagnosis of IFIs is challenging; existing treatments are associated with poor tolerability, drug–drug interactions, TDM requirements, and lack of evidence. Isavuconazole for the treatment of IA and mucormycosis is well-supported by robust clinical evidence. Isavuconazole has a favourable pharmacokinetic and safety profile, which may simplify treatment through reduced drug–drug interactions, and reduced need for TDM.