Since the discovery of epidermal growth factor receptor (EGFR) as a molecular target for non-small–cell lung cancer (NSCLC), first-, second-, and third-generation EGFR tyrosine kinase inhibitors (TKIs) have been developed. However, the optimal sequencing of these agents to maximize the duration of EGFR-TKI treatment has yet to be elucidated. GioTag is a global real-world study investigating sequential treatment with afatinib and osimertinib. Dr Akito Hata, Director of Department of Thoracic Oncology, Kobe Minimally Invasive Cancer Center in Kobe, Japan, discusses the role of afatinib in EGFR mutation-positive NSCLC in light of the GioTag data and shares his clinical experience with the GioTag sequence.
Overview of available EGFR-TKIs
The advent of EGFR TKIs has revolutionized the treatment of
EGFR mutation-positive NSCLC from standard platinum-doublet chemotherapy to personalized, targeted therapy. These targeted agents include the first-generation reversible TKIs, erlotinib and gefitinib; second-generation irreversible ErbB family blockers, afatinib and dacomitinib; and third-generation
EGFR-wild-type sparing, irreversible T790M mutant-specific inhibitor, osimertinib.
Randomized studies indicate that second- and third-generation EGFR TKIs are superior to first-generation EGFR TKIs in terms of progression-free survival (PFS). In LUX-Lung 7, afatinib treatment was associated with a significantly improved PFS (median, 11.0 vs 10.9 months; hazard ratio [HR], 0.73; p=0.017) and time to treatment failure (median, 13.7 vs 11.5 months; HR, 0.73; p=0.0073) compared with gefitinib in chemotherapy-naïve patients with advanced lung adenocarcinoma positive for
EGFR mutations (exon-19 deletions or L858R point mutation). [
Lancet Oncol 2016;17:577-589] In the ARCHER 1050 study, the median PFS with dacomitinib was 14.7 months compared with 9.2 months for participants who received gefitinib (HR, 0.59, 95 percent confidence interval [CI], 0.47–0.74; p<0.0001). With 31.3 months of median follow-up, the median overall survival (OS) was 34.1 months with dacomitinib vs 26.8 months for gefitinib (HR, 0.76; p=0.0438). Notably, this trial excluded patients with brain metastases. [
Lancet Oncol 2017;18:1454–1466] In the FLAURA trial, osimertinib significantly improved PFS vs gefitinib/erlotinib (median, 18.9 vs 10.2 months; HR, 0.46, 95 percent CI, 0.37–0.57; p<0.001). [
N Engl J Med 2018;378:113-125] Final OS was recently reported at the ESMO 2019 Congress, at
a median of 38.6 months for osimertinib vs 31.8 months for gefitinib/erlotinib
(HR, 0.799; p=0.0462). [ESMO 2019, abstract LBA5_PR]
First-line 3rd-generation EGFR TKI vs sequential use of EGFR TKIs
Unfortunately, regardless of which first-line EGFR TKIs is chosen, resistance to therapy inevitably develops. Several mechanisms of acquired resistance have been identified, the most common of which is the T790M mutation in exon 20 of
EGFR, detected in approximately 50–70 percent of patients treated with gefitinib, erlotinib, or afatinib at the time of acquired resistance. [
J Thorac Oncol 2017;12:S2137]. However, there is limited data regarding resistance mechanisms following first-line osimertinib, which appear to be highly heterogenous, generally not well understood or not treatable.
According to Hata, “Japanese lung cancer clinical guidelines recommend osimertinib as first line (Grade 1) for patients with
EGFR mutation-positive NSCLC but other therapeutic options, including afatinib can be proposed. Some patients actually select afatinib in the hope of obtaining a longer oral TKI duration and therefore, chemotherapy-free duration.” He further explained, “For patients on first-line afatinib, whose disease has progressed due to
EGFR T790M mutation, osimertinib will be used in second line, thereby lengthening the oral TKI duration,” said Hata. “However, there is a lack of targeted treatment options following osimertinib failure. Thus, chemotherapy is the most prevalent treatment option after first-line osimertinib.”
“Moreover, from clinical experience, a longer duration of oral TKI therapy appears to be associated with longer survival. After TKI therapy, the survival rates on chemotherapy are similar. Since the survival benefit with chemotherapy is similar, sequential use of EGFR TKIs may be the key to maximizing duration of chemotherapy-free treatment and survival benefit.”
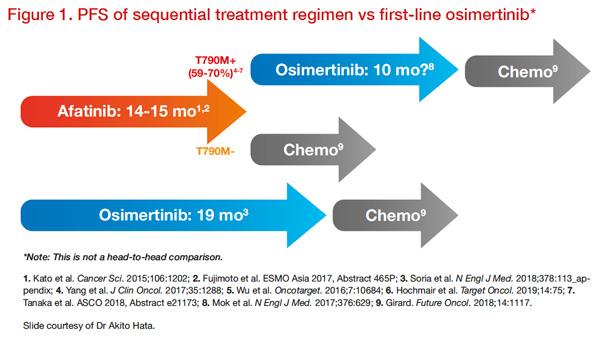
Figure 1 illustrates how sequential treatment may maximize the time on targeted drugs and defer the need for more toxic chemotherapy regimens.
Dr Hata would consider sequential therapy in younger, working patients with a good ECOG PS, who are looking to maximize the duration of chemotherapy-free treatment and the potential survival benefit. Importantly, the patients must understand that osimertinib cannot be used as second-line treatment in T790M-negative disease, and can accept the potential lost opportunity of using osimertinib.
Currently, there is no method to preselect patients who are likely to develop T790M-positive disease after treatment with first- or second-generation EGFR TKI before initiating treatment. However, the majority of patients using afatinib first-line are likely to develop T790M-positive disease, as it is the predominant resistance mechanism following afatinib use. In particular, up to 75 percent of patients with del19 mutations develop T790M+ after first-line afatinib. [
J Clin Oncol 2017;35:1288; Oncotarget 2016;7:10684;
Target Oncol 2019;14:75; ASCO 2018, abstract e21173] Preliminary evidence also suggests that afatinib-resistant subclones are homogenous and likely to be very sensitive to second-line osimertinib. [
Future Oncol 2019;15:637-652]
In an updated analysis of GioTag, sequential treatment with afatinib followed by osimertinib was associated with encouraging OS overall in patients with
EGFR mutation-positive NSCLC (median, 41.3 months, 90 percent CI, 36.8–46.3), and was particularly promising in the subgroup with del19 mutation (median, 45.7 months, 90 percent CI, 45.3–51.5). [
Future Oncol 2019;15:2905-2914]
GioTag study:Remarkable results in Asian patients
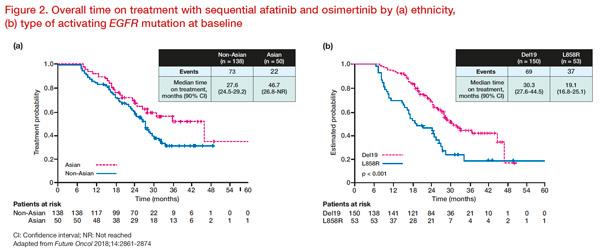
The GioTag study evaluated outcomes of patients who received first-line afatinib followed by osimertinib in a real-world clinical practice setting which included patients that may not have been well-represented in other studies (eg, ECOG PS ≥2, patients with brain metastases). The overall median time on treatment (TOT) — which reflects chemotherapy-free duration — was 27.6 months (90 percent CI, 25.9–31.3). Interestingly, this clinical benefit (TOT) was consistent across all patient subgroups, with exceptionally promising results seen in those with del19-positive disease (median, 30.3 months, 90 percent CI, 27.6–44.5) and Asian patients (median, 46.7 months, 90 percent CI, 26.8–not reached) (
Figure 2). Thus, the median OS for Asians is likely to be at least 46.7 months, although this has yet to be reported. The 2-year OS rate for the overall study population was 78.9 percent. [
Future Oncol 2018;14:2861-2874]
An updated analysis of the GioTag study showed that sequential afatinib and osimertinib conferred OS of almost 3.5 years, and time to treatment failure (TTF) of over 2 years, in a broad population of patients with
EGFR mutation-positive NSCLC. The updated 2-year survival rate was 80 percent for the overall population and 82 percent for patients with del19 mutations. [
Future Oncol 2019;15:2905-2914]“
At present, there are no randomized controlled trials to support sequential therapy rather than upfront third-generation TKIs. However, GioTag, an observational study of first-line afatinib followed by osimertinib, shows great promise especially in Asian patients,” remarked Hata. “Further studies are warranted to compare its effectiveness against upfront third-generation EGFR TKI.”
CASE STUDY: Giotag sequence in clinical practice
Dr Hata shared the following case encountered in his clinical practice:
A 63-year-old, Japanese, female patient was diagnosed with
EGFR mutation-positive (L858R) stage IV lung adenocarcinoma, with metastases in the brain, bone, and lymph nodes. Her ECOG performance status was 1. She was treated with afatinib first-line and showed good response for 11 months (
Figure 3).
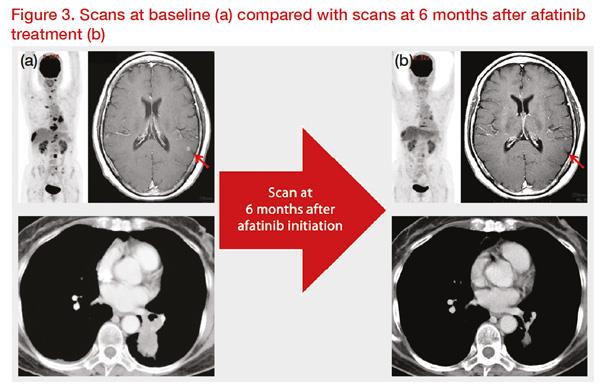 *Clinical images courtesy of Dr Akito Hata.
*Clinical images courtesy of Dr Akito Hata.
Then, progressive disease (PD) was discovered on the primary site. A rebiopsy using bronchoscopy was conducted, and T790M-positive disease was confirmed after 1 month. Second-line osimertinib treatment was administered. Good response was observed for about another 11 months, after which isolated brain metastases were found. Bone and lymph node lesions remained stable. Using response evaluation criteria in solid tumours (RECIST), PD after second-line treatment was confirmed. Osimertinib was continued for 8 months beyond PD, and the isolated brain metastases were treated with stereotactic radiation therapy using cyberknife (
Figure 4).
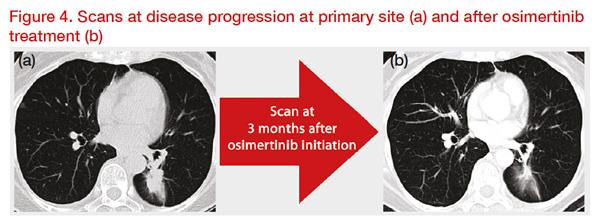 *Clinical images courtesy of Dr Akito Hata.
*Clinical images courtesy of Dr Akito Hata.
Overall, the patient’s PFS was 11 months, the time to treatment failure was 19 months, and the time on targeted therapies (afatinib and osimertinib), ie, the chemotherapy-free duration, was 31 months. These results are impressive considering the patients’ baseline condition.
 *Clinical images courtesy of Dr Akito Hata.
*Clinical images courtesy of Dr Akito Hata. *Clinical images courtesy of Dr Akito Hata.
*Clinical images courtesy of Dr Akito Hata.