Systemic therapy plays a central role in the management of unresectable hepatocellular carcinoma (HCC). In this rapidly developing therapy area, recent clinical trials are redefining systemic treatments, including multiple kinase inhibitors (MKI). During the recent Gastro Lunch and Learn sessions sponsored by Eisai, Dr David Tai and Dr Yong Wei Peng discussed the evidence behind MKIs such as lenvatinib (Lenvima®) in the treatment of unresectable HCC through a case-based approach.
Updates in the treatment landscape of unresectable HCC
The treatment of unresectable HCC has evolved over the last decade with the emergence of various classes of systemic therapies. Tai said guidelines now recommend oral MKIs such as sorafenib and lenvatinib for first-line treatment, whereas immunotherapy (ie, nivolumab) may be considered for those who cannot tolerate MKIs.
Tai discussed the results of REFLECT*, the landmark study that supported the use of lenvatinib in the first-line setting. REFLECT was an open-label, phase III, multicentre, noninferiority trial that included 954 patients with unresectable HCC, who had not received treatment for advanced disease. Patients were treated with either lenvatinib (12 mg daily for bodyweight ≥60 kg or 8 mg daily for bodyweight <60 kg) or sorafenib 400 mg twice daily in 28-day cycles. The median overall survival (OS) for the lenvatinib-treated patients was 13.6 months (95 percent confidence interval [CI], 12.1–14.9), which was noninferior to sorafenib (12.3 months, 95 percent CI, 10.4–13.9; hazard ratio [HR], 0.92, 95 percent CI, 0.79–1.06). [
Lancet 2018;391:P1163-1173]
Tai further explained that approximately one-third of all patients in either arm of REFLECT received subsequent anticancer medication during follow-up. The median overall OS for patients treated initially with lenvatinib, followed by any subsequent anticancer medication, was 21 months. [
J Clin Oncol 2019;37[4_Suppl]:371-371] In contrast, the median OS for patients who received sorafenib, followed by subsequent anticancer medication, was 17 months.
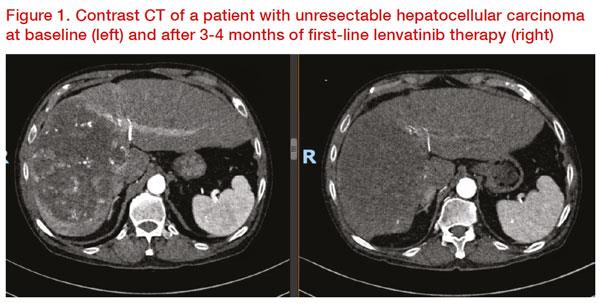
He then presented the case of a 70-year-old male patient with a 16 x 10 cm mass on the right lobe of liver segments 7, 8, and 5 extending to segment 4 on CT scan. Contrast CT showed arterial phase heterogeneous hyperenhancement, intratumoural aneurysms, venous and delayed phase hypodense washout. The patient underwent yttrium-90 transarterial radioembolization (TARE), which resulted in a partial response per mRECIST. Six months after TARE, the patient’s Child-Pugh (CP) score was 5 and his albumin-bilirubin (ALBI) score was -2.32 (Grade 2). Lenvatinib was initiated subsequently, which resulted in further reduction in tumour enhancement consistent with treatment effect (
Figure 1). Eventually, the patient was able to undergo extended right hepatectomy. Histopathology revealed moderately differentiated (G2) HCC of the right liver with vascular invasion and prominent treatment effect with 90 percent tumour necrosis. Tai concluded that this case is a good example of tumour response to lenvatinib, which allowed downstaging and eventual surgical resection.
Systemic therapy for HCC
European guidelines, for example, the European Association for the Study of the Liver (EASL) and the European Society for Medical Oncology (ESMO) strongly recommend the use of MKIs such as lenvatinib and sorafenib in the treatment of patients with advanced HCC (Barcelona Clinic Liver Cancer [BCLC] stage C) and well-preserved liver function, with no preference for one over the other.
However, Yong noted that this may not be the case in the Asia-Pacific region. He shared that during the Asia-Pacific Primary Liver Cancer Expert Meeting 2019, the consensus recommendations on the use of MKIs in advanced HCC considered lenvatinib as the preferred MKI for the first-line treatment of advanced HCC (BCLC C) among Asians. He explained that based on the subgroup analysis of REFLECT*, lenvatinib had progression-free survival (PFS) advantage over sorafenib, which was more prominent in patients from the Asia-Pacific region. In particular, these patients had a 39 percent lower risk of disease progression or death vs sorafenib-treated patients (median PFS, 7.3 months vs 3.6 months with sorafenib; HR, 0.61, 95 percent CI, 0.51–0.73). In contrast, among Western patients, lenvatinib treatment was associated with a similar risk of progression or death vs sorafenib (median PFS, 7.4 months vs 5.5 months with sorafenib; HR, 0.81, 95 percent CI, 0.61–1.08). [
J Clin Oncol 2017;35[suppl]: Abstract 4001]
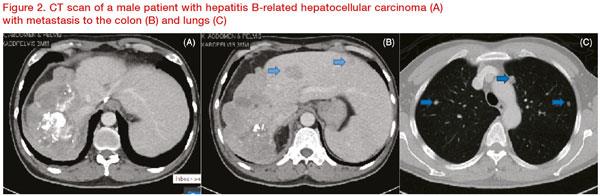
Yong then presented the case of a 63-year-old Chinese male patient with hepatitis B-related HCC who had undergone four sessions of transarterial chemoembolization (TACE) and radiofrequency ablation over the span of 5 years. Eventually, the patient’s disease progressed to BCLC C. The patient had good liver function (CP A), an alpha-fetoprotein level of 64 ng/mL, and good performance status (PS 1). However, CT scan revealed bowel and lung metastasis (
Figure 2).
Yong emphasized that this patient is a suitable candidate for lenvatinib therapy, having several characteristics that suggest a favourable treatment outcome. Aside from being Chinese, the patient’s disease is also associated with hepatitis B. Subgroup analysis of REFLECT* also showed that in patients with hepatitis B, lenvatinib was associated with a 38 percent lower risk of progression or death compared with sorafenib (median PFS, 7.3 months vs 3.6 months, respectively; HR, 0.62, 95 percent CI, 0.50–0.75). [
J Clin Oncol 2017;35[suppl]:Abstract 4001]
Yong added that the adverse effect profiles of the two MKIs were generally similar, although lenvatinib had a higher rate of hypertension (42 percent vs 30 percent for sorafenib) whereas sorafenib had a higher rate of palmar-plantar erythrodysaesthesia (52 percent vs 27 percent for lenvatinib).
In conclusion, he underscored that survival of patients with advanced HCC has been significantly prolonged with the availability of systemic therapies like MKIs, which remain the guideline-recommended first-line treatment. He also noted that the role of immunotherapy is rapidly evolving, particularly the combination of anti-vascular endothelial growth factor receptors and immunotherapy for advanced HCC appeared promising.
*REFLECT: A Multicenter, Randomized, Open-Label, Phase 3 Trial to
Compare the Efficacy and Safety of Lenvatinib (E7080) Versus Sorafenib
in the First-Line Treatment of Subjects With Unresectable Hepatocellular
Carcinoma.