The three most common functional gastrointestinal (GI) disorders are
regurgitation, infantile colic, and constipation. At a recent symposium,
Professor Hania Szajewska from the Department of Paediatrics at the
Medical University of Warsaw, Poland, discussed the role of probiotics,
specifically
Lactobacillus reuteri DSM 17938 (
Lactobacillus reuteri Protectis) in managing these disorders.
Prevalence and impact of functional GI disorders in infants
Functional GI disorders (FGIDs) are chronic or recurrent GI symptoms not explained by structural or biochemical abnormalities. The three most frequent FGIDs in infants are regurgitation, infantile colic, and constipation, with a worldwide prevalence of approximately 30, 20, and 15 percent, respectively. [
BMJ Open 2016;6:e011475;
J Pediatr Gastroenterol Nutr 2015;61:531-537] Symptoms of FGID include vomiting, problematic feeding (eg, spitting, regurgitation), inconsolable or persistent crying, and hard stools or straining.
Colic in infants leads one in six families (17 percent) to consult a health professional. [
BMJ Clin Evid 2015;2015:0309] Infantile colic is defined as crying and or fussing for more than 3 hours per day, for more than 3 days per week, over a period of at least 7 days in infants less than 5 months of age, with no evidence of infant failure to thrive, fever, or illness. “These recurrent and prolonged periods of infant crying, fussing, or irritability occur without obvious cause, and cannot be prevented or resolved by caregivers,” explained Szajewska. “Though infantile colic is considered benign, it is a source of concern and frustration.”
Moreover, colic is strongly associated with maternal depression, child abuse (shaken baby syndrome), and early breastfeeding cessation. [
Acta Paediatr 2009;98:1344-1348;
Proc Natl Acad Sci USA 2012;109 Suppl 2:17294-17301;
Breastfeed Med 2006;1:146-155] Limited evidence suggests that it may contribute to medium- and long-term outcomes, such as later sleep problems, family dysfunction, and behavioural problems. [
Acta Paediatr Suppl 2005;94:129-132;
Pediatrics 1995;96(1 Pt 1):43-47;
Acta Paediatr 2000;89:13-17]
Probiotics for FGID: Lactobacillus reuteri DSM 17938
Probiotics have been shown to be beneficial in a number of clinical situations, but not all probiotics are equal, emphasized Szajewska. Similar to antibiotics, the effects of probiotics are both strain- and clinical-condition specific.
Lactobacillus reuteri DSM 17938 (
Lactobacillus reuteri Protectis) has been widely studied in over 70 studies in children, in various conditions including infantile colic, regurgitation, acute gastroenteritis, constipation, diarrhoea, and abdominal pain, among others, said Szajewska. For infantile colic,
L. reuteri DSM 17938 is the only probiotic studied in more than one trial, with multiple randomized controlled trials (RCTs) clocked to date. [
Pediatrics 2010;126:e526-e533;
J Pediatr 2013;162:257-262;
BMJ 2014;348:g2107;
J Pediatr 2015;166:74-78;
Antonie Van Leeuwenhoek 2015;107:1547-1553;
J Pediatr 2017;191:170-178.e2;
Pediatrics 2018;141:e20171811]
L. reuteri DSM 17938 works on FGID by reducing dysbiosis, and decreasing inflammation and visceral pain, while improving gut motility.
Role of Lactobacillus reuteri DSM 17938 in infantile colic
There is accumulating evidence that the gut microbiota in infants with colic differs from that of healthy controls. The microbial signatures of infants with colic at 7 or 14 postnatal days showed slower colonization, lower diversity and stability, reduced butyrate-producing species, increased proteobacteria (including species producing gas and inflammation), and reduced lactobacilli and bifidobacteria (including species with anti-inflammatory effects). [
Gut Microbes 2013;4:416-421] In addition, colic is linked with gut inflammation (as determined by faecal calprotectin) and dysbiosis, independent of mode of feeding, with fewer Bifidobacilli. [
J Pediatr 2018;203:55-61.e3.]
Whereas data on other probiotics are limited, evidence supports the efficacy of
L. reuteri DSM 17938 in the management of infantile colic. Multiple clinical trials have demonstrated that
L. reuteri reduces the symptoms of infantile colic (crying or fussing) in breastfed babies. [
Pediatrics 2010;126:e526-e533;
J Pediatr 2013;162:257-262;
J Pediatr 2015;166:74-78;
Antonie Van Leeuwenhoek 2015;107:1547-1553;
Pediatrics 2018;141:e20171811] Treatment success was defined as a reduction of at least 50 percent in daily average crying time. A systematic review of RCTs found that a daily dose of 10
8 CFU was associated with treatment success (relative risk, 1.67, 95 percent confidence interval [CI], 1.10–2.81; number needed to treat [NNT] 5, 95 percent CI, 4–8) and reduced crying time at the end of the intervention. [
Arch Med Sci 2018;14:1137-1143] Moreover, a separate individual participant data meta-analysis found an increased success rate (28 percent vs 9 percent; NNT=4) and decreased daily crying time by 25.4 minutes in the probiotic group vs the placebo group. [
Pediatrics 2018;141:e20171811]
Studies also investigated if probiotics given to healthy babies may help to prevent infantile colic. In two RCTs involving 574 infants,
L. reuteri DSM 17938 reduced crying time by 44 minutes vs placebo. [
Cochrane Database Syst Rev 2019 Mar 13;3:CD012473]
Role of Lactobacillus reuteri DSM 17938 in regurgitation
In a randomized controlled study,
L. reuteri DSM 17938 was shown to reduce regurgitation episodes, reduce gastric distension, and improve gastric motility in infants with functional regurgitation. [
Eur J Clin Invest 2011;41:417-422] A double-blind RCT investigated the effects of a formula containing partially hydrolysed whey protein, starch, and
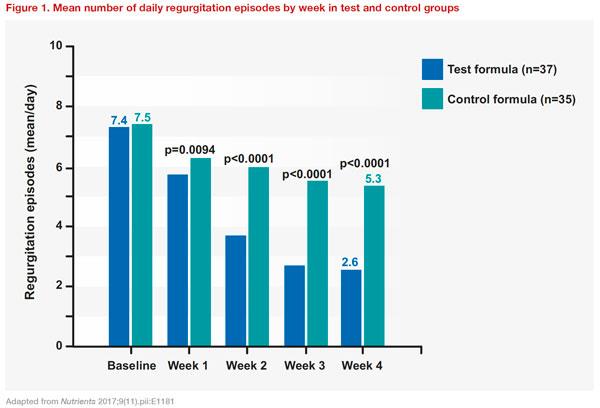
L. reuteri DSM 17938 (test formula) on gastric emptying rate in infants with regurgitation, compared with a standard infant formula (control formula). A total of 80 infants aged 4 weeks to 5 months were randomized to receive either test or control formula. Compared with controls, the test group showed greater percentage changes in gastric emptying rate (12.3 percent vs 9.1 percent; p<0.01), and mean daily regurgitations were significantly decreased (test: 7.4 at baseline to 2.6 at week 4; control: 7.5 at baseline to 5.3; between group difference: p<0.0001)
(Figure 1). [
Nutrients 2017;9(11).pii:E1181]
Role of Lactobacillus reuteri DSM 17938 in FGID prevention
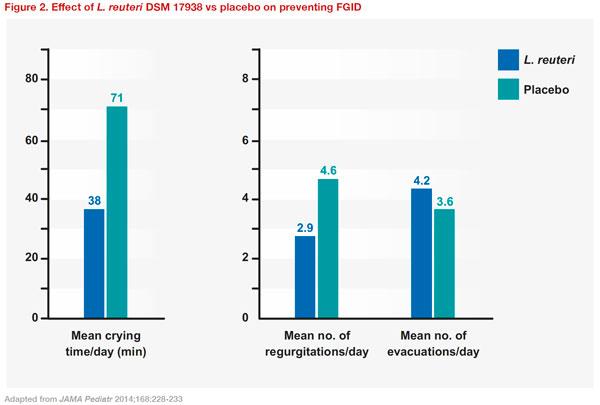
A prospective, multicentre, double-masked, placebo-controlled RCT was conducted in term infants to evaluate whether oral supplementation with
L. reuteri DSM 17938 during the first 3 months of life can reduce colic, regurgitation, and constipation. A total of 589 infants were randomized to receive either
L. reuteri DSM 17938 or placebo daily from day 3 for 90 days. At 3 months of age, the use of
L. reuteri DSM 17938 significantly improved the mean duration of crying time (38 vs 71 minutes; p<0.01), the mean number of regurgitations per day (2.9 vs 4.6; p<0.01), and the mean number of evacuations per day (4.2 vs 3.6; p<0.01), compared with placebo
(Figure 2). The authors concluded that supplementation with
L. reuteri DSM 17938 could represent a new therapeutic strategy for preventing FGID in infants. In addition, the study found that prophylactic use of
L. reuteri DSM 17938 reduced private and public costs for the management of FGIDs. [
JAMA Pediatr 2014;168:228-233]
An earlier study investigated the effect of
L. reuteri supplementation on feeding tolerance and GI motility in healthy formula-fed preterm infants. Results showed that newborns receiving probiotics showed a significant decrease in regurgitation and mean daily crying time and a larger number of stools compared with those given placebo. Gastric emptying rate was significantly increased, and fasting antral area was significantly reduced in both the newborns receiving
L. reuteri and breast-fed newborns compared with placebo. These findings suggest a useful role for
L. reuteri supplementation in formula-fed preterm newborns. [
J Pediatr 2008;152:801-806]
Key takeaways
FGIDs are common in infants. Though considered benign, FGIDs are a significant source of concern and frustration for caregivers. Microbiota is likely to play a role in FGIDs, particularly in infantile colic. However, more hard data are needed. There is evidence of efficacy of
L. reuteri in the management of some FGIDs, including infant colic, regurgitation, and constipation. Notably, the effects of probiotics are strain- and clinical-condition specific, and data on other probiotics are limited.