 The Wingspan stent (image courtesy of Stryker)
The Wingspan stent (image courtesy of Stryker)Is stenting appropriate for this subgroup of ICAD patients who do not respond to medical therapy? The Wingspan Stent System Post Market Surveillance (WEAVE) study showed that selected ICAD patients may derive significant benefit from intracranial stenting when used according to approved criteria. Four experts, Prof Miao Zhong Rong (China), Prof Yu Chun Ho (Hong Kong), Prof Seo Jung Hwa (South Korea), and Prof Wong Ho Fai (Taiwan), share their perspectives on the WEAVE trial and the role of stenting in ICAD.

ICAD: Overview and prevalence
ICAD occurs when atherosclerotic plaque builds up in major intracranial arteries, causing stenosis. It can result in thromboembolism and subsequent transient or permanent cerebral ischaemic events and is a major cause of ischaemic stroke worldwide. ICAD is more common in the Asian, African, and Hispanic populations than in Caucasian individuals. [J Neuroimaging 2009;19:11S-16S] Among those of Chinese ethnicity, the incidence ranges between 30 and 50 percent of patients with ischaemic stroke. [Stroke 1993;24:779-786]The Chinese Intracranial Atherosclerosis (CICAS) Study found that 46.6 percent of patients with stroke had intracranial occlusive disease. [Stroke 2014;45:663-669] “This could be extrapolated into an annual incidence of approximately 810,000 of the 2.5 million strokes in China, assuming 70 percent of strokes in China are ischaemic,” said Miao. Wong and Yu both estimated the prevalence of ICAD in their respective regions of Hong Kong and Taiwan to be 30 percent for ischaemic stroke, while Seo put the figure for South Korea at 40 percent.
The WEAVE trial
The WEAVE trial is a post-market surveillance trial mandated by the US FDA to assess the periprocedural safety of the Wingspan Stent system (Stryker, Kalamazoo, MI) in the treatment of symptomatic ICAD. It is the largest on-label, multicentre, prospective trial of the Wingspan stent system to date. [Stroke 2019;50:889-894]
The trial enrolled a total of 152 consecutive patients from 24 hospitals who met the FDA on-label usage criteria for the Wingspan stent, such as age (22–80 years), symptomatic intracranial atherosclerotic stenosis of 70–99 percent, baseline modified Rankin Scale score ≤3, ≥2 strokes in the vascular territory of the stenotic lesion with at least 1 stroke while on medical therapy, and stenting of the lesion ≥8 days after the last stroke. Patients underwent angioplasty and stenting with the Wingspan stent, a self-expanding nitinol stent used in coordination with the use of the Gateway angioplasty balloon. The primary analysis assessed the periprocedural stroke, bleed, and death rate within 72 hours of the procedure with adjudication by a core study Stroke Neurologist.
The results showed that following on-label indications resulted in an extremely low periprocedural stroke and death rate of 2.6 percent with Wingspan. This was better than the FDA periprocedural event safety benchmark of 4 percent. Of the 152 patients, 148 (97.4 percent) were event-free at 72 hours, 2 (1.3 percent) had nonfatal strokes, and 2 (1.3 percent) patients died. [Stroke 2019;50:889-894]
Importantly, high volume clinical experience of the interventionalists with the Wingspan stent is critical in obtaining low complication rates. Interventionalists who had experience of >50 Wingspan cases before enrolling their first patient in the WEAVE study had a zero percent (0/69 stent cases) periprocedural stroke and death index event rate in the on-label cases they enrolled in the trial, whereas interventionalists with <50 Wingspan cases before the trial had a 4.8 percent (4/83 stent cases) index event rate.
The authors concluded that with experienced interventionalists and proper patient selection following the on-label usage guidelines, the use of the Wingspan stent for ICAD led to a low periprocedural complication rate and favourable safety profile. [Stroke 2019;50:889-894]
Experts' perspective
Significance of the WEAVE trial“The WEAVE trial is important to neurologists as it identifies the target population for Wingspan stent treatment and addresses perioperative safety issues,” said Miao. Yu agreed: “The WEAVE trial showed that stenting for ICAD is not as dangerous as SAMMPRIS showed,” referring to the Stenting versus Aggressive Medical Therapy for Intracranial Arterial Stenosis trial. [N Engl J Med 2011;365:993–1003] This trial evaluated patients in an extended clinical application of the Wingspan stent, beyond its approved FDA labelling. This included stenting patients who had not failed medical therapy, those who may have presented with transient ischaemic attacks only, without history of stroke, and stenting patients earlier than 8 days after their qualifying event. Notably, the majority of patients stented in the SAMMPRIS trial did not meet the on-label indication, which resulted in a periprocedural stroke, bleed, and death rate of 14.7 percent. [N Engl J Med 2011;365:993–1003] In contrast, in the WEAVE trial, the periprocedural event rate was just 2.6 percent for on-label patients (Table). [Stroke 2019;50:889-894]

“The SAMMPRIS trial showed high periprocedural complication rate for ICA stenting. This is a large barrier to perform intracranial artery stenting. Fortunately, the WEAVE trial showed that this high periprocedural complication can be reduced depending on the time of ICA stenting and the experience of ICA stenting. These results opened up the possibility that stent treatment may be reintroduced to ICAD treatment,” said Seo.
Since its introduction in 2005, the design of the Wingspan stent has not changed substantially except for modifications of the radio-opaque markers. Therefore, the WEAVE results imply that the high periprocedural seen in the SAMMPRIS trial were unlikely to be due to issues with the stent itself, but probably stemmed from a combination of factors, such as interventionalists’ lack of experience, poor patient selection, and underdeveloped standards of practice in intracranial stenting. “The different results from the WEAVE trial vs SAMMPRIS make neurologists more confident to consider the use of the Wingspan stent to treat selected ICAD patients,” said Wong.
Stenting: Indications, referral and considerations
In clinical practice, ICA stenting is usually considered only if secondary prevention fails in aggressive medical management, in symptomatic patients with severe intracranial artery stenosis, with hypoperfusion mechanism infarction, and poor collateral circulation.
All experts agreed that the timing of the stenting after a stroke event affects the periprocedural outcomes. In addition, interventionalists should consider other factors such as percentage of stenosis, parent artery size, collateral circulation, lesion length, calcification, target vessel, anatomy, and morphology of the vessel and the patient’s condition. They shared clinical case examples of patients who had undergone stenting successfully.
Clinical case examples
Case 1: Chronic symptomatic R-MCA almost total occlusion(Contributed by Prof Miao Zhong Rong)
A 45-year-old man presented with chronic aphasia and left hemiparesis. His right middle cerebral artery (R-MCA) showed 85 percent stenosis. He was treated with medical therapy, but it was not effective. Hence, he was referred for angioplasty and stenting.
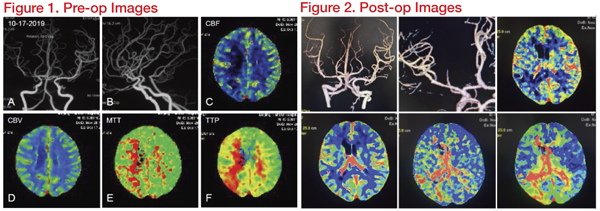
In this case, the middle and distal segments of the middle cerebral artery were severely narrowed, and the stenosis was adjacent to the bifurcation, but did not spread to the upper and lower trunk openings. The undersized balloon (Gateway1.5*15mm) was chosen, following the open cell stent with accurate deployment. A 2.5*15mm Wingspan stent was delivered to the lesions. The guide wire landing area was selected to be the lower trunk, which is smooth and has a larger diameter. This reduced the damage of the branching vessels due to the expansion of the bifurcation. After the stent was implanted, the lumen became thinner at the proximal end of the stent. We consider vasospasm to be the cause. After intravenous injection of nimodipine, the angiogram showed quick relief. In addition, the lenticulostriate artery is centred on the medial side, and the opening has a certain anatomical distance from the proximal end of the stenosis. The risk of perforating complications after intravascular interventional therapy is relatively low.
Case 2: ICAD stenosis close to a branch origin
(Contributed by Prof Yu Chun Ho)
A 53-year-old man experienced recurrent episodes of right-sided weakness lasting for 5 minutes each time. The patient has hypertension (BP 190/108 mm Hg) and hypercholesterolaemia (total lipoprotein 6.7mmol/L, LDL 4.1mmol/L) and was on multiple antihypertensive medication, anticholesterol drug, and double antiplatelet therapy.
Digital subtraction angiography (DSA) revealed 85 percent short segment stenosis at left MCA, M1 just proximal to the origin of a temporal branch (Figure 3). The risk of occlusion of the origin of the temporal branch due to plaque displacement as a result of angioplasty was considered.
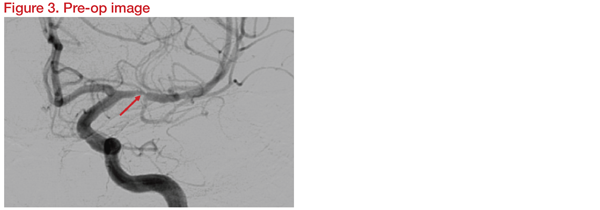
Three-Dimensional Rotational Angiography (3DRA) was carried out for assessment in detail and planning. Instruments planned for the procedure were the Gateway balloon 1.5*15mm and Wingspan stent 2.5*9mm. Following the intervention, DSA and 3DRA showed preservation of the temporal branch origin (Figure 4).
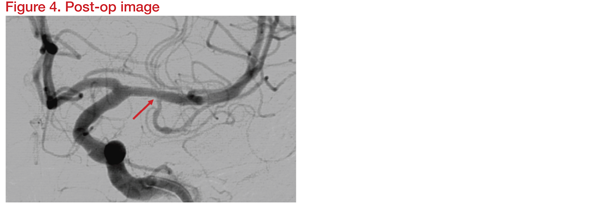
At 12 months, there was no evidence of branch thrombosis or in-stent restenosis. The patient was well and symptom free. This showed that angioplasty and stenting using Gateway and Wingspan is safe for M1 ICAD stenosis close to a temporal branch origin without delayed branch thrombosis or in-stent restenosis.
Case 3: Symptom progression
(Contributed by Prof Seo Jung Hwa)
An 80-year-old presented with left hemiparesis and dysarthria and NIH stroke scale (NIHSS) of 7. She was diagnosed with an acute infarction involving right basal ganglia and frontoparietal area and severe stenosis involving right distal ICA, probable cavernous segment. The patient was treated with dual antiplatelet/high dose statin. Six days later, the symptoms worsened with altered mentality. The NIHSS score deteriorated from 7 to 14. IA thrombectomy using Trevo stent was performed. Though the residual stenosis remained, the right cavernous ICA was recanalized, and the patient improved to 7 NIHSS points (Figure 5).
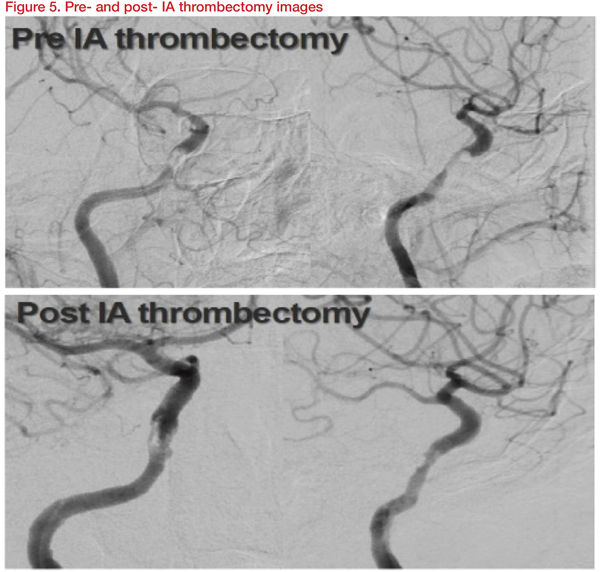
The patient was maintained with dual antiplatelet and high-dose statins. The patient again deteriorated to NIHSS score 13 later, after the IA thrombectomy. Definite restenosis was observed in cerebral angiography. Stenting was carried out with a Gateway balloon 3.5*15mm and Wingspan 4*20mm stent. After stenting, the patient improved to NIHSS Score 8 (Figure 6).
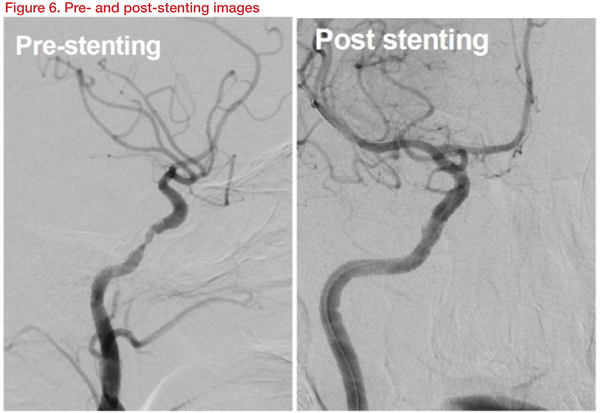
This case showed that even with medical management for stenosis, if symptoms worsen and restenosis is observed, stenting can help prevent the recurrence of symptoms and restenosis.
Case 4: Type II PIA stenosis with contralateral intracranial VA occlusion
(Contributed by Prof Wong Ho-Fai)
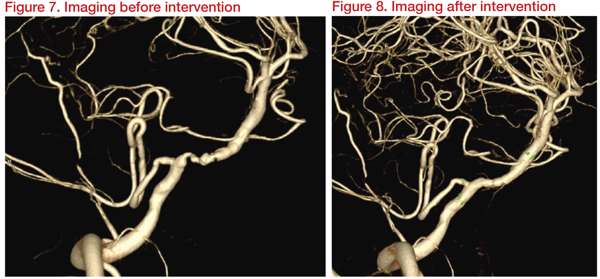
A 61-year-old man presented with drowsiness, speech disturbance, and four-limb weakness, with NIHSS 20 and Modified Rankin Scale (mRS) 5. His clinical history includes hypertension and smoking. He was diagnosed with basilar artery occlusion (BAO) with acute infarction and ICAD with bilateral vertebral arteries occlusion.
Medication with dual antiplatelet treatment (DAPT) and percutaneous transluminal angioplasty (PTA) with intracranial stenting was planned. Three weeks after DAPT, imaging results revealed a Type II proatlantal intersegmental artery (PIA) with 66 percent stenosis at right vertebral artery (VA, V4 segment) distal to posterior inferior cerebellar artery (PICA). Hypoplasia of left vertebrobasilar junction and occlusion of left VA with plaque was observed.
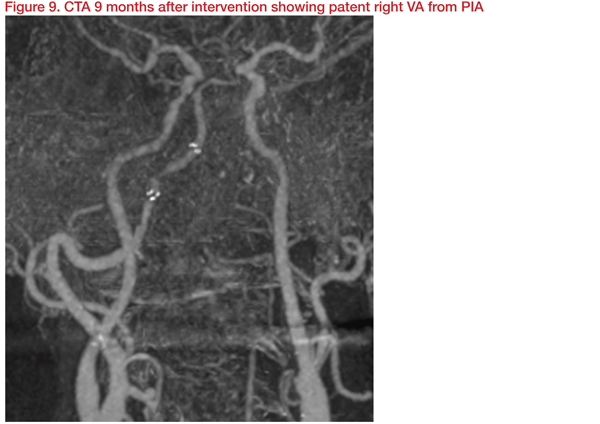
Angioplasty and stenting were carried out with Gateway 3*15mm and Wingspan 4*20mm. This achieved dilatation of right VA (PIA) from 1.2 mm to 2.4 mm and restoration of basilar flow. At discharge, clinical muscle power improved, and 10 months later, NIHSS and mRS improved to 13 and 3, respectively.
Key takeaways
The WEAVE trial results suggest re-evaluation of stenting as a treatment option for symptomatic patients with ICAD is needed, given the better safety profile than earlier reports and that patients suffering from ICAD may benefit from endovascular treatment with the Wingspan Stent System.Proper patient selection and sufficient experience on the part of interventionalists are key to successful outcomes in intracranial stenting.