Three direct-acting antivirals (DAAs) have been linked to rare cases of severe liver injury in some patients treated for chronic hepatitis C virus (HCV) infection, the US Food and Drug Administration (FDA) has warned.
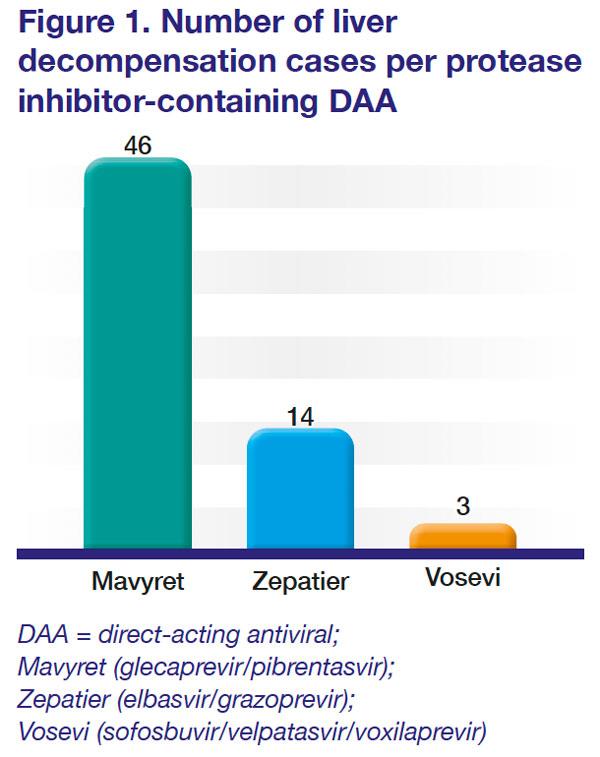
Sixty-three cases of liver decompensation, including liver failure and death, were identified among those treated with glecaprevir/pibrentasvir (Mavyret, n=46), elbasvir/grazoprevir (Zepatier, n=14), and sofosbuvir/velpatasvir/voxilaprevir (Vosevi, n=3). [
Figure 1] The cases were reported to the FDA Adverse Event Reporting System (FAERS) database or identified in the medical literature until the first week of 2019. [https://www.fda.gov/drugs/drug-safety-and-availability/fda-warns-about-rare-occurrence-serious-liver-injury-use-hepatitis-c-medicines-mavyret-zepatier-and; accessed 10 December 2019]
All three DAAs are FDA-approved to treat chronic hepatitis C. Sofosbuvir/velpatasvir/voxilaprevir is particularly indicated for adult patients with chronic HCV infection without liver impairment or with mild liver impairment (Child-Pugh A).
However, the cases reported to the agency were apparently concentrated on patients who had evidence of advanced liver disease. “Liver failure occurred in those who had signs and symptoms of moderate-to-severe liver impairment (Child-Pugh B or C) or other serious liver problems [who] should not have been treated with these medicines,” the FDA said in a drug safety communication.
“Some cases occurred in patients who were misclassified as having no cirrhosis or compensated cirrhosis with mild liver impairment (Child-Pugh A) prior to therapy but had evidence of a more severe liver disease (ie, decreased platelets, portal hypertension). Others had pre-existing risk factors such as liver cancer, alcohol abuse, or serious medical illnesses associated with serious liver problems, which might have contributed to the worsening of liver function or liver failure during treatment,” the FDA said.
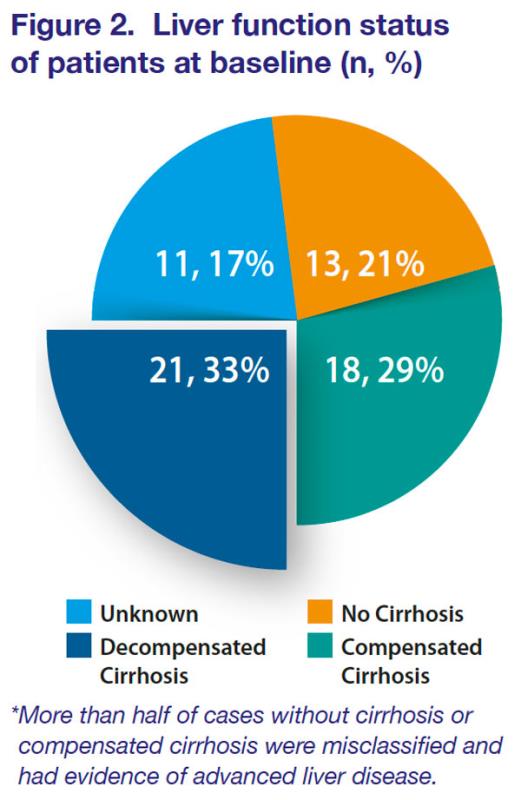
Of the 63 cases reported, 13 (21%) were in patients without cirrhosis, 18 (29%) were in those with compensated cirrhosis, 21 (33%) had decompensated cirrhosis, and 11 (17%) had unknown liver function status at baseline. [
Figure 2]
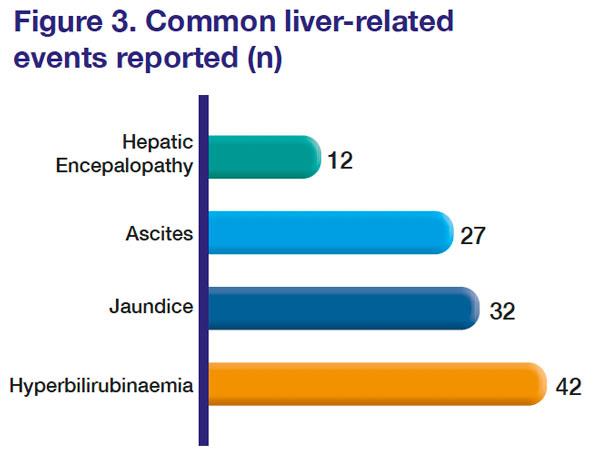
The median time to onset of a liver-related event, or liver decompensation post-treatment initiation, was 22 days (range, 2 days–16 weeks). Hyperbilirubinaemia was the most common liver-related event, followed by jaundice, ascites, and hepatic encephalopathy. [
Figure 3] In most patients, symptoms resolved or new onset worsening of liver function improved after stopping the medicine.
Safe and effective when prescribed as indicated
The FDA assured clinicians that the three DAAs remained generally “safe and effective”, when used in patients without liver impairment or with only mild liver impairment. Healthcare professionals should continue to prescribe these drugs for the right patients.”
The agency however advised that liver disease severity should be assessed at baseline, and any signs or symptoms of worsening liver function (increases in liver enzymes, jaundice, ascites, encephalopathy, and variceal haemorrhage) be closely monitored, particularly in patients with pre-existing liver problems or risk factors (liver cancer or alcohol abuse). Clinicians are encouraged to report any potential adverse events to the FDA.
In addition, patients should be aware that the risk of serious liver injury is rare, the FDA said. However, should they experience fatigue, weakness, loss of appetite, nausea and vomiting, or notice yellow eyes or skin, or light-colored stools while taking the drug, they should consult their physician immediately as these may be signs of liver injury.