Osteoporosis a common disorder among
postmenopausal women that results in an increased risk of fractures requires
long-term therapy. In a recent symposium in Singapore, Professor Peter Ebeling
discussed the concept of goal-directed osteoporosis therapy, the therapeutic
benefits of denosumab in fracture risk reduction, and the consequences of
treatment discontinuation and drug holidays.
Hormones play a central role in the bone mass changes that occur with age, particularly in women. Peak bone mass is typically achieved from puberty to early adulthood, after which the bone mineral density (BMD) declines with age. During menopause, bone loss accelerates and then continues to progress in postmenopausal women, albeit at a slower pace. With advancing age, BMD loss leads to microarchitectural deterioration of the bone, increased fragility, and consequently, elevated fracture risk.
1
The diagnosis of the osteoporosis is established by measuring the BMD or by the occurrence of adulthood hip or vertebral fracture in the absence of major trauma.
2 In postmenopausal women and men aged 50 and older, the World Health Organization defines osteoporosis as having a BMD value of 2.5 standard deviations (SD) or more below the reference standard (T-score of ≤-2.5); those with a T-score of ≤-2.5 and had at least one fracture are diagnosed as severe osteoporosis.
2 In premenopausal women and men below the age of 50, however, the International Society for Clinical Densitometry recommends ethnic- or race-adjusted Z-scores instead of T-scores; Z-scores of -2.0 or lower defined as either “low BMD for chronological age” or “below the expected range for age” and those above -2.0 being “within the expected range for age”.
3
Goal-directed therapy for osteoporosis
Prior to initiating treatment for osteoporosis, the goal of treatment for the patient needs to be established as the initial choice of treatment, as well as the decisions to stop, continue, or change treatment, would be based on the achievement or progress towards achieving that goal. The overriding goal of treatment is to achieve freedom from fracture, or at least, to reduce the risk of fracture.
“Most clinicians would initiate treatment in patients with hip or vertebral fractures, patients with a T‐score of ≤ –2.5 in the lumbar spine, total hip or femoral neck, and those with ≥3 percent 10‐year probability of hip fracture or ≥20 percent 10‐year probability of a major osteoporotic fracture using FRAX
®,” said Ebeling.
If a fracture occurs despite increases in BMD or favourable responses in bone turnover markers, a switch to a more potent anti-osteoporotic treatment, or to a combination of treatments may be warranted. “In cases like these, an incident fracture trumps T-scores, ie, treatment should be continued regardless of the T‐score because the risk of another fracture in the next few years is very high,” he added.
Effective fracture risk reduction with denosumab
Among the pharmacologic options for the long-term treatment of postmenopausal osteoporosis include the receptor activator of nuclear factor kappa-B (RANK) ligand (RANKL) inhibitor, denosumab. Denosumab is primarily indicated for individuals with osteoporosis at high risk of fracture, particularly women at the postmenopausal stage or those receiving an aromatase inhibitor for breast cancer, as well as men receiving androgen deprivation therapy.
4
The Fracture REduction Evaluation of Denosumab in Osteoporosis every 6 Months (FREEDOM) trial has shown that in women with osteoporosis, denosumab effectively reduced the relative risk of new vertebral, nonvertebral, and hip fractures by 68 percent, 20 percent and 40 percent, respectively, compared with placebo.
5
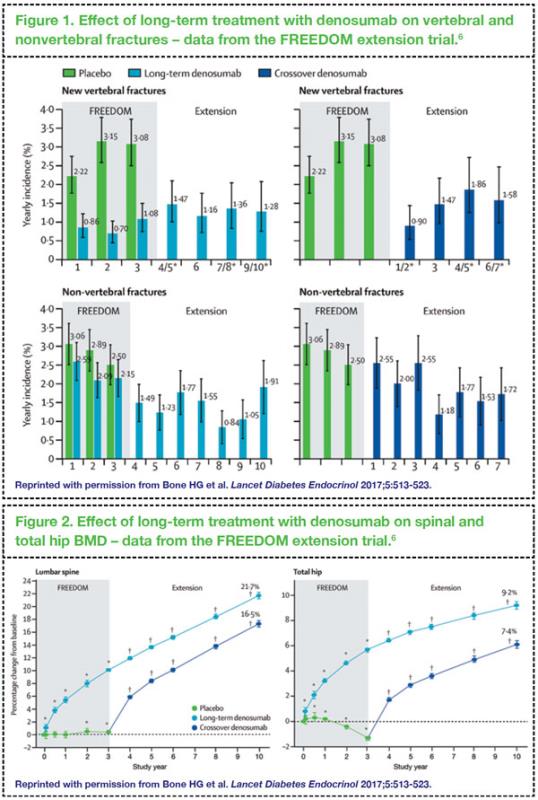
In the open-label, 7-year extension phase of this study, long-term treatment with denosumab for up to 10 years has been shown to be associated with low rates of adverse events, sustained reduction in the incidence of vertebral and nonvertebral fractures, and continued increases in spinal and total hip BMD (
Figures 1 and 2).
6 Notably, the benefits on BMD and fracture risk reduction were also seen in those who received 3 years of placebo and transitioned to denosumab in the extension study.
Switching to denosumab
Switching to denosumab may be considered in patients who experience significant bone loss or a fracture following bisphosphonate therapy for more than 12 months; in those who experience adverse events or intolerance to oral bisphosphonates; in those with adherence issues or find bisphosphonate dosing inconvenient; or in cases where bisphosphonates are contraindicated.
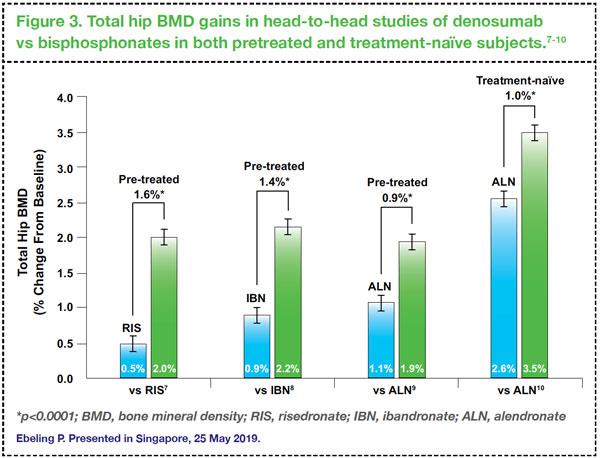
In head-to-head studies comparing denosumab versus bisphosphonates, switching to denosumab has consistently resulted in further BMD gains in total hip BMD compared with continuing treatment with bisphosphonates in women who received prior treatment with either risedronate, ibandronate or alendronate (
Figure 3).
7,8,9 In another trial involving treatment-naïve subjects, similar findings were reported: denosumab showed significantly larger gains in BMD and greater reduction in bone turnover markers compared with alendronate at 12 months.
10
No drug holidays for denosumab
Rare, but serious safety concerns – such as osteonecrosis of the jaw and atypical femoral fractures – have been raised regarding the long-term use of antiresorptive agents such as bisphosphonates and denosumab. Although the risks of these adverse events are very low, a drug holiday may be considered in patients receiving bisphosphonates after 3–5 years of treatment if they are not at high fracture risk; these patients should be reassessed 2–3 years after discontinuation.
11
However, in patients receiving denosumab, a drug holiday is not recommended as BMD gains with even long-term denosumab therapy can rapidly reverse upon discontinuation of treatment.
12 In an observational study, patients who received denosumab for 8 years and those who received placebo for 4 years followed by denosumab for 4 years showed continuous improvements in lumbar spine and total hip BMD. After discontinuing denosumab for 1 year, however, BMD rapidly decreased (6.7 percent loss per year in those who received denosumab for 8 years and 5.1 percent loss per year in those who transitioned from placebo to denosumab at year 4).
13 Also, in the FREEDOM extension trial, vertebral fracture risk increased in patients who discontinued denosumab, although not to levels greater than those seen in patients who received and then discontinued placebo.
14
Based on these data, patients on denosumab should therefore cancel the drug holiday, or transition to an alternative antiresorptive, eg, oral bisphosphonate, if denosumab treatment is to be discontinued. Data suggest that follow-on therapy with alendronate and zoledronic acid after discontinuation with denosumab may be able to sustain some of the BMD gains observed while patients were on denosumab, and may thus confer some protective effect against fractures.
15,16,17
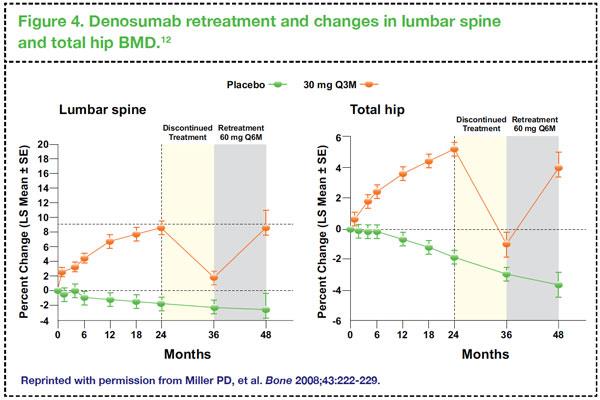
It is reassuring to note that in patients who discontinue denosumab, treatment can be restarted, with consequent gains in BMD. In a phase II study in women with low BMD, it has been demonstrated that retreatment with denosumab following a period of discontinuation can raise lumbar spine and total hip BMD to levels similar to those seen during the initial denosumab treatment (
Figure 4).
12
Conclusions
Denosumab is effective in reducing vertebral, nonvertebral, and hip fractures. Transitioning from bisphosphonates to denosumab results in further increases in BMD. Drug holidays are not appropriate for denosumab because of its rapid reversibility of action and loss of BMD.
References:
1. Kanis JA, et al.
Osteoporos Int 2002;13:527-536.
2. Cosman F.
Osteoporos Int 2014;25:2359-2381.
3. International Society for Clinical Densitometry. 2013 Official Positions—Adult. Available at: http://www.iscd.org/official-positions/2013-iscd-official-positions-adult/. Accessed 05 June 2019.
4. Prolia
® (denosumab) Prescribing Information, accessed 05 June 2019.
5. Cummings SR, et al.
N Engl J Med 2009;361:756-765.
6. Bone HG et al.
Lancet Diabetes Endocrinol 2017;5:513-523.
7. Roux C, et al.
Bone 2014;58:48-54.
8. Recknor C, et al.
Obstet Gynecol 2013;121:1291-1299.
9. Kendler DL, et al.
J Bone Miner Res 2010;25:72-81.
10. Brown JP et al.
J Bone Miner Res 2009;24:153-161.
11. Adler RA, et al.
J Bone Miner Res 2016; 31:16-35.
12. Miller PD, et al.
Bone 2008;43:222-229.
13. McClung M, et al.
Osteoprosis Int 2017;28:1723-1732.
14. Cummings SR, et al.
J Bone Miner Res 2018;33:190-198.
15. Freemantle N, et al.
Osteoporos Int 2012;23:317-326.
16. Lehmann T, Aeberli D.
Osteoporos Int 2017;28:3067-3068.
17. Reid IR, et al.
Calcif Tissue Int 2017 Oct;101:371-374.
Sponsored as a service to the medical profession by Amgen.