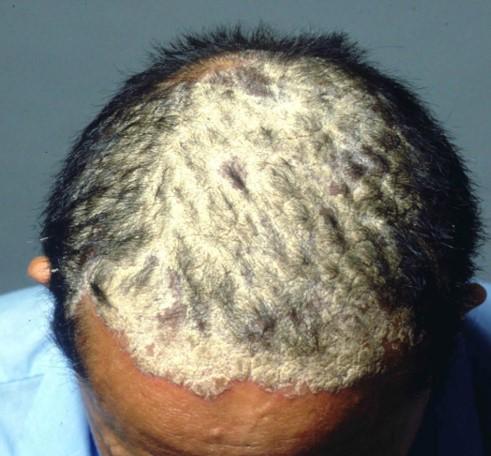
 Psoriasis of the scalp
Psoriasis of the scalpApremilast is an effective treatment option for moderate-to-severe psoriasis of the scalp, a new trial has found.
The phase IIIb, double-blind, placebo-controlled trial included 252 patients who were randomly assigned to receive apremilast (n=168) or placebo (n=84). Only those with moderate-to-severe disease who showed inadequate response to at least one prior therapy were eligible. The primary efficacy endpoint was the proportion of patients showing Scalp Physician Global Assessment (ScPGA) response.
After 16 weeks of treatment, significantly more patients in the apremilast group achieved the primary endpoint relative to the placebo comparators (43.3 percent vs 13.7 percent; adjusted difference, 29.6 percent, 95 percent confidence interval [CI], 19.5–39.7; p<0.0001). Apremilast was given as 30-mg, twice-daily doses.
The comparative advantage of apremilast over placebo emerged as early as week 2 and was retained at all time points through week 16.
Moreover, a significantly greater proportion of apremilast vs placebo patients achieved a ≥4-point improvement on the scalp itch numerical rating scale (NRS; 47.1 percent vs 21.1 percent; p<0.0001). Change was assessed relative to baseline values. The same was true for whole body itch NRS (45.5 percent vs 22.5 percent; p<0.0001).
A total of 302 patients were given at least one dose of the study medication and were thus included in the safety analysis. The most common adverse events (AEs) in the apremilast group were diarrhoea, nausea, headache, and vomiting, all of which occurred substantially more frequently than in placebo counterparts.
Two patients in the apremilast group had serious AEs, five had severe AEs, and 11 experienced AEs that led to drug withdrawal.