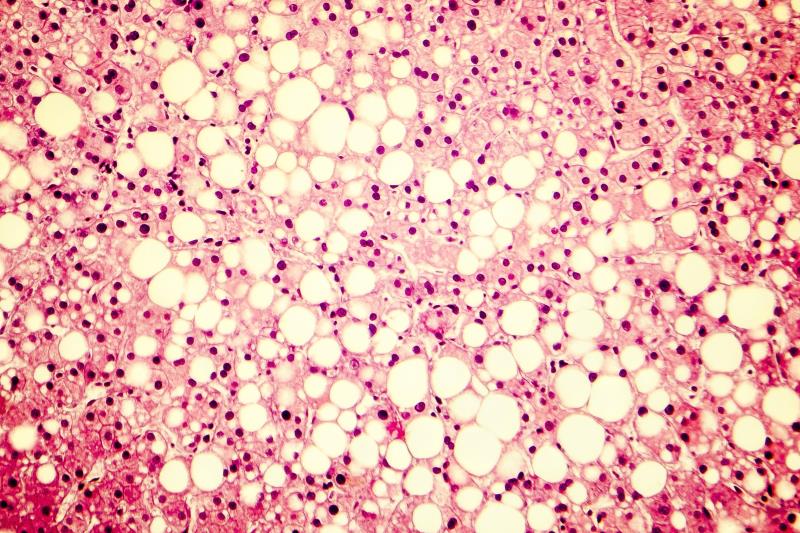
Use of aldafermin, an engineered analogue of FGF19, appears beneficial in the treatment of patients with nonalcoholic steatohepatitis (NASH), reducing liver fat and markers of disease progression without any adverse side effects, according to the results of a phase II trial.
Seventy-eight patients (mean age, 53.5 years; 54 percent female; 62 percent had type 2 diabetes) were randomized to receive treatment with aldafermin 1 mg (n=53) or placebo (n=25) for 24 weeks. Stage 3 fibrosis was present in 45 percent and 41 percent patients in the respective groups. All patients had absolute liver fat content (LFC) of ≥8 percent, as measured by magnetic resonance imaging-proton density fat fraction.
Compared with placebo, 24 weeks of treatment with aldafermin led to a significant reduction in absolute LFC (–7.7 percent vs –2.7 percent; difference, –5.0 percent; p=0.002). Moreover, the drug yielded significantly greater decreases in levels of 7alpha-hydroxy-4-cholesten-3-one, bile acids, alanine and aspartate aminotransferases, and Pro-C3.
A higher number of patients receiving aldafermin vs placebo achieved fibrosis improvement (≥1 stage) with no worsening of NASH (38 percent vs 18 percent; p=0.10), as well as NASH resolution with no worsening of fibrosis (24 percent vs 9 percent; p=0.20).
None of the patients in the active treatment group withdrew due to adverse events as opposed to 4 percent in the placebo group.
Aldafermin inhibits bile acid synthesis and regulates metabolic homeostasis, and the present data contribute to the growing body of evidence on the potential of drug in treating NASH, researchers said.