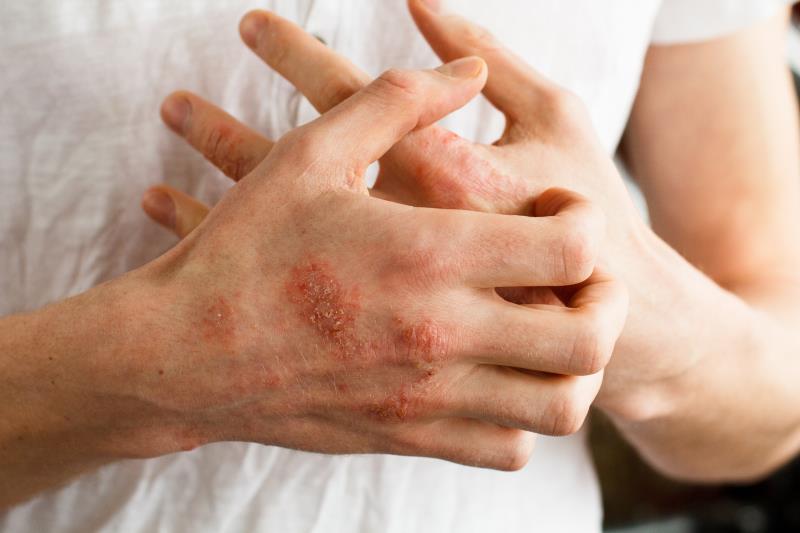
Crisaborole treats atopic dermatitis (AD) by suppressing skin inflammation and improving barrier function, a recent study has found.
Forty adults (mean age, 32.2 years; 27 women) with mild-to-moderate AD were randomly allocated, on a 1:1 basis, to the crisaborole or vehicle control group for 14 days. This was followed by a 28-day open-label phase where crisaborole was given to all participants to apply to all affected areas. Punch biopsies were collected at days 8 and 15 for biomarker analysis.
Lesional total sign score showed a greater 15-day change for lesions that were treated with crisaborole than with the vehicle control (–4.5 vs –2.1 points; p<0.0001). This effect was present as early as day 8 (–3.3 vs –1.2 points; p<0.0001).
Similarly, better pruritus numerical rating scale scores were reported for the crisaborole-treated lesions. This was evident starting from the first day after treatment initiation and remained consistent until the 15-day follow-up.
These clinical benefits could be explained by the molecular changes that crisaborole effected. Compared with vehicle-treated lesions, the mRNA expression profiles of inflammatory mediator genes were significantly improved in areas treated with crisaborole. This included the suppression of key AD-related genes and enrichment of negative regulators.
“Moreover, only crisaborole-treated lesions showed a reversal in molecular dysregulation, approaching the nonlesional skin profile at both time points for the entire study population and for each patient,” said researchers, pointing out that changes in vehicle-treated regions were more modest and could be explained off as potential effects of the ointment base.