
ABSTRACT
Preterm birth (PTB) is birth that occurs before 37 weeks’ gestation, and it is a leading cause of perinatal morbidity and mortality.1 Babies born before 34 weeks’ gestation are particularly associated with high rates of morbidity and mortality.2 They are also at risk of long-term medical and social sequelae.3 It is therefore important to institute preventative measures that can help mitigate the occurrence of PTB in pregnant women. This review highlights the various phenotypes of spontaneous PTB, risk factors, and pathophysiological pathways associated with the syndrome. It also discusses the various screening and preventative measures currently employed by clinicians, such as transvaginal sonographic screening for a short cervix, progesterone therapy, placement of cervical cerclage, insertion of cervical pessary, antibiotic treatment for lower genital tract infections, and tocolytic therapy, while exploring professional guidelines in the prevention of spontaneous PTB.
INTRODUCTION
PTB is defined as birth that occurs before 37 weeks’ gestation. It is the leading cause of death in neonates and the second cause of death in children aged <5 years.1 PTB alone is estimated to cause over a million neonatal deaths per year worldwide. The highest risk of perinatal mortality occurs in babies born before 32 weeks’ gestation. Prematurity-associated complications include respiratory distress syndrome, necrotizing enterocolitis, intraventricular haemorrhage, and developmental brain disorders, such as cerebral palsy, intellectual disabilities, and vision impairment. In adult life, those who are born preterm are challenged with noncommunicable diseases including obesity, diabetes mellitus, and hypertension.4
CLASSIFICATIONS OF PRETERM BIRTH
PTB is classified as spontaneous or iatrogenic. Spontaneous preterm labour and preterm premature rupture of membranes (PPROM) account for about 70% and 40% of all PTBs in singleton and twin pregnancies, respectively. Iatrogenic PTB usually emanates from serious complications in pregnancy such as preeclampsia and foetal growth restriction. Given the inverse association between the risk of perinatal morbidity and mortality and gestational age at birth, PTB is further stratified according to gestational age at delivery: extreme PTB (<28 weeks), early PTB (28–33+6 weeks), and late PTB (34–36+6 weeks).1 This review would focus on prevention strategies for spontaneous PTB.
STRATEGIES FOR PREVENTION OF PRETERM LABOUR
Risk factors
Multiple risk factors are identifiable before and during pregnancy. Increased risk of spontaneous PTB has been associated with the following risk factors: history of PTB, maternal age at >40 years, teenage pregnancy, obesity, poor weight gain during pregnancy, African ethnicity, a short inter-pregnancy interval of <6 months, conception by assisted reproductive technology, multiple pregnancies, substance abuse, cigarette smoking, low socioeconomic status, bacterial vaginosis, periodontal disease, and a history of excision of the cervical transformational zone.5
Methods for identification of high-risk women
Spontaneous PTB is a complex syndrome, making it difficult to prevent. One major challenge for the prevention of this adverse outcome relates to the ability to effectively identify high-risk women. Although PTB history is the most important risk factor, this alone has been shown to have limited predictability, with detection rates (DR) ranging from 15–30%.6 Despite this limitation, it is the only method of screening recommended by most professional obstetrical societies.7A cohort study including 34,025 singleton pregnancies has reported that a prior episode of spontaneous preterm delivery increases the risk of spontaneous PTB in the index pregnancy by 6-fold, and the risk is increased by 20-fold when there are two prior episodes of spontaneous preterm delivery. However, screening by maternal risk factors can only detect 38.2% of preterm deliveries in women with previous pregnancies at or beyond 16 weeks and 18.4% in those without, at 10% false-positive rate.8 There is a need to consider other screening methods.
Transvaginal ultrasonographic assessment of cervical length at 16–24 weeks’ gestation (Figure 1) was shown to be a useful predictor of spontaneous PTB,9 either alone or in combination with maternal risk factors. A study of 6,819 women with singleton pregnancy reported that 111 women (1.6%) had a short cervix of <15 mm and 294 women (4.3%) had funnelling at 22–24 weeks’ gestation. The prevalence of funnelling decreased (from 98% to 25% and <1%) with increasing cervical length from ≤15 mm to 16–30 and >30 mm, respectively. Women with funnelling showed a significantly higher rate of preterm delivery at <33 weeks’ gestation than those without (6.9% vs 0.7%; p=0.0001).10 A large prospective observational study of 58,807 singleton pregnancies demonstrated that the combination of cervical length at 20–24 weeks’ gestation and maternal risk factors achieved DR of 80.6%, 58.5%, 53.0%, and 28.6% for extreme (<28 weeks), early (28–30 weeks), moderate (31–33 weeks), and mild (34 weeks) spontaneous PTB, respectively.11
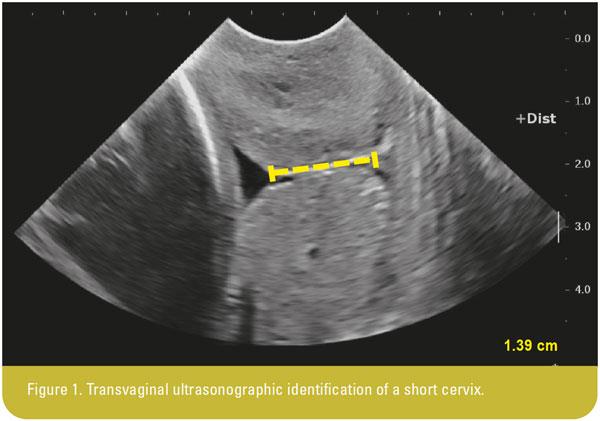
In relation to multiple pregnancies, a different cut-off for the measurement of cervical length is required for the prediction of spontaneous PTB. A study of 215 women with twin pregnancies demonstrated that a cervical length of ≤25 mm at 23 weeks’ gestation achieved DR of 100%, 80%, 47%, and 35%, respectively, for spontaneous delivery at <28, <30, <32, and <34 weeks’ gestation. The respective DR for cervical length of ≤15 mm was 50%, 40%, 24%, and 11%.12 In a study of 51 triplet pregnancies, a cervical length of ≤25 mm at 15–20 weeks’ gestation had DR of 100%, 72%, and 25% for spontaneous delivery at ≤28, <30, and ≤32 weeks’ gestation, respectively.13
The cervicovaginal fluid (CVF) reflects the biochemical environment and physiological changes of the vagina, cervix, and adjacent overlying foetal membranes. Therefore, it has been the main biological fluid used to determine biomarkers that could predict spontaneous PTB. Two CVF biomarkers – foetal fibronectin and phosphorylated insulin-like growth factor binding protein – are used as point-of-care tests to predict PTB in women presenting with threatened preterm labour. However, the predictive ability of these biomarkers for spontaneous PTB in asymptomatic women is poor to moderate. Thus, the search for predictive CVF biomarker continues.14
Methods of prevention
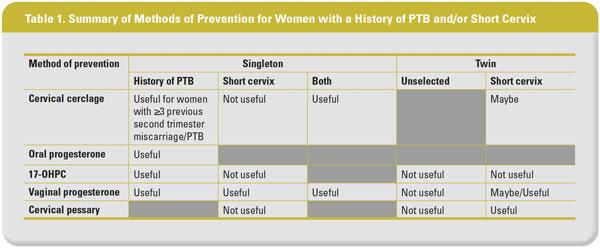
For women with history of PTB and/or a short cervical length, there are essentially three preventive measures – cervical cerclage, progesterone therapy, and cervical pessary (Table 1).
Cervical cerclage
In the general obstetric population, approximately 0.5–1% of pregnant women have been found to have an incompetent cervix. Such women may report a history of early or mid-trimester pregnancy loss, which is often associated with painless cervical dilatation.7 Cerclage is a circumferential suture carefully placed in the cervix, with the aim to provide mechanical support to the cervix and thereby reduce the risk of PTB.
A landmark randomized controlled trial (RCT) conducted by the Royal College of Obstetricians and Gynaecologists evaluated 1,292 women whose obstetricians were uncertain whether to recommend cervical cerclage. Majority of the participants had a history of PTB or cervical surgery. The findings showed that the application of cervical cerclage decreased the rate of delivery at <33 weeks’ gestation from 17% to 13% (odds ratio [OR], 0.72, 95% confidence interval [CI], 0.53–0.97). This risk reduction is mainly attributed to the effect of cervical cerclage on women with prior history of ≥3 second trimester miscarriage or PTB compared with <37 weeks’ gestation (15% vs 32%; OR, 0.37, 95% CI, 0.14–0.95). These findings led to the recommendation that cervical cerclage should be offered to women at high risk of PTB, such as those with a history of ≥3 pregnancies ending before 37 weeks’ gestation.15 The Cervical Incompetence Prevention Randomized Cerclage Trial (CIPRACT) evaluated 67 women with a history of PTB at <34 weeks’ gestation, demonstrated no significant difference in the rate of PTB at <34 weeks’ gestation between the cerclage and expectant management groups (13.0% vs 13.6%; OR, 0.95, 95% CI, 0.21–4.21). However, in the subgroup of women with a short cervix of <25 mm, the rate of PTB at <34 weeks’ gestation was significantly reduced in the cerclage group vs the expectant management group (10.0% vs 62.5%; OR, 0.07, 95% CI, 0.01–0.82).16
In relation to isolated short cervical length of <25 mm, in the absence of history of PTB, a recent individual patient-level data (IPD) meta-analysis of five RCTs involving 419 asymptomatic singletons reported no significant difference in the rate of PTB at <35 weeks’ gestation between women with and without cerclage (21.9% vs 27.7%; RR, 0.88, 95% CI, 0.63–1.23).17 With regard to women with previous history of PTB with concurrent short cervix (<25 mm), a meta-analysis of five trials including 504 women with singleton pregnancy demonstrated that cerclage was associated with reduced rates of preterm birth at <35 weeks’ gestation (30% reduction; RR, 0.70, 95% CI, 0.55–0.89) and perinatal mortality and morbidity (36% reduction; RR, 0.64, 95% CI, 0.45–0.91).18
A recent Cochrane review and meta-analysis reported on the effect of cervical cerclage in singleton pregnancy at high risk of pregnancy loss based on woman’s history and/or ultrasound finding of a short cervix. Results showed that pregnant women with cerclage are less likely to have PTB at <34 weeks (average RR, 0.77, 95% CI, 0.66–0.89) and <37 weeks (average RR, 0.80, 95% CI, 0.69–0.95; 9 studies; n=2,415).19
Cervical cerclage was also evaluated in twin gestations. However, existing evidence is rather controversial. An IPD meta-analysis comprising three trials with 49 twin gestations with a short cervix of <25 mm identified before 24 weeks’ gestation showed that women who received cervical cerclage (n=24) had a similar rate of PTB at <34 weeks’ gestation compared with those who did not receive (n=25; 62.5% vs 24.0%; adjusted odds ratio [adjOR], 1.17, 95% CI, 0.23–3.79).20 Based on the results of this meta-analysis, cervical cerclage is not recommended for clinical use in twin gestations where the mother has a short cervical length in the second trimester. However, results from a recent meta-analysis of 16 studies (RCT and cohort studies) on 1,211 twin pregnancies with a cervical length of <15 mm have refuted this recommendation. The meta-analysis showed that cervical cerclage was significantly associated with prolongation of pregnancy (mean difference, 3.8 weeks, 95% CI, 2.2–5.6) and reduction in the rates of PTB at <34 (RR, 0.57, 95% CI, 0.43–0.75) and <37 weeks’ gestation (RR, 0.86, 95% CI, 0.74–0.99) compared with the no cerclage group,21 suggesting that cervical cerclage might be beneficial for women with twin pregnancy with a short cervix of <15 mm.
Progesterone therapy
Labour begins when there is a decrease in progesterone and an upsurge in oestrogen concentration or when progesterone activity is halted, resulting in ripening of the cervix and uterine contractility. Progesterone functions by preventing cervical ripening, reducing myometrial contractility through the suppression of oxytocin receptor synthesis and regulation of inflammation. Several RCTs have assessed the possible effect of exogenous progesterone intake, including oral intake, weekly intramuscular injections of 17α-hydroxyprogesterone caproate (17-OHPC), and daily vaginal progesterone, in asymptomatic women at risk of spontaneous PTB.22-25
A recent meta-analysis of three RCTs comprising a total of 386 singleton pregnancies with history of spontaneous PTB showed that those who received oral progesterone compared with placebo had a significant decrease in the risk of preterm delivery at <37 (42% vs 63%; RR, 0.68, 95% CI, 0.55-0.84; p=0.0005) and <34 weeks’ gestation (29% vs 53%; RR, 0.55, 95% CI, 0.43-0.71; p=0.00001). Conversely, an increase in gestational age at delivery was observed in women taking oral progesterone vs placebo (mean difference, 1.71 weeks, 95% CI, 1.11–2.30).25
With regards to the use of 17-OHPC in women with a history of miscarriage/PTB, a meta-analysis of seven RCTs including 630 singleton pregnancies with a history of ≥2 miscarriages and/or PTBs concluded that 17-OHPC was effective as a prophylactic agent for PTB, with a pooled OR of 0.50 (95% CI, 0.30–0.85).26 In an RCT of 659 pregnant women with singleton pregnancy with a history of spontaneous PTB, the authors reported no significant difference in the rate of PTB at <32 weeks’ gestation between the vaginal progesterone (100 mg/day) and placebo groups (RR, 0.9, 95% CI, 0.52–1.56).27 On the contrary, a similar trial that included 142 high-risk singleton pregnancies with a history of PTB, prophylactic vaginal progesterone (100 mg/day) reportedly reduced the rate of preterm delivery at <34 (2.8% vs 18.6%; p=0.002) and <37 weeks’ gestation (13.8% vs 28.5%; p=0.03) compared with placebo.28
The efficacy of weekly 17-OHPC and daily vaginal progesterone from 16 weeks’ gestation in the prevention of spontaneous PTB was compared in a meta-analysis of three RCTs with a total of 680 singleton pregnancies with prior spontaneous PTB. The study reported that vaginal progesterone was associated with lower rates of spontaneous PTB at <34 (RR, 0.71, 95% CI, 0.53–0.95) and <32 weeks’ gestation (RR, 0.62, 95% CI, 0.40–0.94) compared with 17-OHPC, leading to the conclusion that daily vaginal progesterone administered from 16 weeks’ gestation is a better alternative than weekly 17-OHPC in preventing spontaneous PTB in women with history of spontaneous PTB.24
Evidence on the use of 17-OHPC in preventing PTB in women identified with a short cervix but no history of PTB is less convincing. A multicentre RCT compared weekly intramuscular 250 mg 17-OHPC with placebo in 657 nulliparous women with singleton pregnancy with a short cervix of <30 mm (corresponding to 10th percentile) identified at 16–22 weeks’ gestation. This study demonstrated no difference between 17-OHPC and placebo in the rates of PTB at <32 (8.6% vs 9.7%; RR, 0.88, 95% CI, 0.54–1.43), <35 (13.5% vs 16.1%; RR, 0.84, 95% CI, 0.58–1.21), and <37 weeks of gestation (25.1% vs 24.2%; RR, 1.03, 95% CI, 0.79–1.35).29 Similarly, in an open-label, multicentre RCT of 105 singleton pregnancies with a cervical length of <25 mm and a history of PTB, cervical surgery, uterine malformation, or prenatal DES exposure, there was no significant difference between the weekly intramuscular 500 mg 17-OHPC and control arms in terms of enrolment-to-delivery interval (77 vs 74 days, mean difference 4 days, 95% CI, -9 to -17) and the rate of delivery at <37 weeks (45% vs 44%; RR, 1.01, 95% CI, 0.66–1.55).30
In relation to the use of vaginal progesterone in preventing PTB among women identified with a short cervix, two major RCTs have provided convincing evidence. A multicentre RCT of 413 women with a short cervix of ≤15 mm at 22 weeks’ gestation demonstrated a significantly lower rate of spontaneous PTB at <34 weeks’ gestation in the vaginal progesterone group vs the placebo group (19.2% vs. 34.4%; RR, 0.56, 95% CI, 0.36–0.86). Moreover, the authors found that vaginal progesterone might be more beneficial for the subgroup with a cervical length of ≥12 mm.22 A similar trial of 458 women with singleton pregnancy and a short cervical length of 10–20 mm at 19–23 weeks’ gestation demonstrated a lower rate of PTB at <33 weeks’ gestation in women who received vaginal progesterone than those on placebo (8.9% vs.16.1%; RR, 0.55, 95% CI, 0.33–0.92).23
Despite promising results on the efficacy of vaginal progesterone in preventing spontaneous PTB, a large RCT of 1,228 women (the OPPTIMUM study) showed no significant effect in the prevention of foetal death or PTB at <34 weeks’ gestation (OR, 0.86, 95% CI, 0.61–0.22) or neonatal outcome (OR, 0.72, 95% CI, 0.44–1.17). The inclusion criteria for this trial were previous spontaneous PTB at <34 weeks’ gestation, a short cervix of ≤25 mm, or a positive foetal fibronectin test at 22–24 weeks with other clinical risk factors for PTB. The negative results may be attributed to several reasons: the randomized women represented a heterogeneous group with different underlying causes for PTB, the moderate overall compliance of 69%, and the lack of power to detect any significant differences in preventing PTB between vaginal progesterone and placebo arms in the subgroup of women with short cervix.31 A recent IPD meta-analysis that included data from the OPPTIMUM study and four other double-blind, placebo-controlled trials evaluated 974 asymptomatic women with singleton pregnancies and a short cervix of ≤25 mm (vaginal progesterone [n=498] and placebo [n=476]). The authors reported that vaginal progesterone significantly reduced the risk of spontaneous PTB at <33 (12% vs 17%; RR, 0.70, 95% CI, 0.51–0.95) and <34 weeks’ gestation (15% vs 20% RR, 0.72, 95% CI, 0.55–0.95), and composite neonatal morbidity and mortality (RR, 0.59; 95% CI, 0.38–0.91).32
Progesterone was also compared with cervical cerclage in the prevention of PTB. A recent meta-analysis including 10 trials (n=769; five trials, vaginal progesterone vs placebo [n=265] and five trials, cerclage vs no cerclage [n=504]) indirectly compared the use of vaginal progesterone and cervical cerclage in women with singleton pregnancies with a history of spontaneous PTB and a short cervix of <25 mm at 16–24 weeks’ gestation. Compared with placebo, vaginal progesterone decreased the risk of PTB at <32 (RR, 0.60, 95% CI, 0.39–0.92) and <35 weeks’ gestation (RR, 0.68, 95% CI, 0.50–0.93), as well as composite perinatal morbidity and mortality (RR, 0.43, 95% CI, 0.20–0.94). Cervical cerclage was also shown to reduce the risk of PTB at <32 (RR, 0.66, 95% CI, 0.48–0.91) and <35 weeks’ gestation (RR, 0.70, 95% CI, 0.55–0.89), as well as composite perinatal morbidity and mortality (RR, 0.64, 95% CI 0.45-0.91) compared with no cerclage. The authors concluded that both vaginal progesterone and cervical cerclage are effective in preventing PTB.33
Some trials have evaluated the use of progesterone in preventing PTB in unselected twin pregnancies or twin pregnancies with a short cervix. Individual trials have demonstrated negative results. An IPD meta-analysis of six RCTs including 303 women with twin pregnancies identified with a sonographic short cervix of ≤25 mm (n=159 and 144 assigned to vaginal progesterone and placebo/no treatment, respectively), reported significant reduction in the risk of PTB at <33 weeks’ gestation (31.4% vs 43.1%; RR, 0.69, 95% CI, 0.51–0.93) and in composite neonatal morbidity and mortality (RR, 0.61, 95% CI, 0.34–0.81).34 While the results suggest that vaginal progesterone reduces the risk of PTB and neonatal morbidity and mortality in women with twin pregnancies and a sonographic short cervix, further research is required before conclusive advice can be provided.
Cervical pessary
Emerging evidence suggests that Arabin cervical pessary could potentially prevent spontaneous PTB in women identified with a short cervix. The Arabin cervical pessary is a silicone ring, which comes in different sizes with the outer ring diameter varying between 64 and 70 mm, inner ring diameter between 32 and 35 mm, and height of the curvature between 21 and 25 mm. The inner ring notches against the cervix and the outer ring fixes the cervix against the pelvic floor. The pessary is designed with the aim to adjust the angle of the cervix towards the posterior wall of the vagina. The device is considered noninvasive and user-friendly. It can be administered in an outpatient setting without anaesthesia and can easily be removed when required.
In a trial that evaluated the treatment effect of the Arabin pessary (n=192) vs expectant management (n=193) in women with singleton pregnancy and a short cervical length of ≤25 mm, a significant reduction in the rate of spontaneous PTB at <34 weeks’ gestation was observed in the treatment group compared with the expected management group (6% vs 27%; OR, 0.18, 95% CI, 0.08–0.37).35 However, subsequent trials failed to replicate the results.36-37 A meta-analysis of three RCTs including 1,612 singleton pregnancies with a cervical length of ≤25 mm at 18–24 weeks’ gestation reported no significant difference between pessary and expectant management in terms of spontaneous PTB rate at <34 weeks’ gestation (RR, 0.51, 95% CI, 0.19–1.38). However, the rate of spontaneous PTB at <37 weeks’ gestation was reduced in the pessary group (RR, 0.46, 95% CI, 0.28–0.77).38
In a global, open-label, multicentre trial including 1,180 unselected twin pregnancies, compared with expectant management, cervical pessary given at 20–24 weeks’ gestation was not associated with a significant reduction in the rate of spontaneous PTB at <34 weeks’ gestation (RR, 1.05, 95% CI, 0.79–1.41).39 In another open-label, multicentre, randomized trial in a similar population, there was no significant difference between the pessary (n=401) and expectant management groups (n=407) in terms of the rate of a composite of poor perinatal outcome (13% vs 14%; RR, 0.98, 95% CI, 0.69–1.39). In a post hoc analysis of women with cervical length of <25th percentile (<38 mm), use of the pessary led to a significant reduction in the rate of poor perinatal outcomes (12% vs 16%; RR, 0.40, 95% CI, 0.19–0.83), but not spontaneous PTB at <28 (4% vs 2%; RR, 2.02, 95% CI, 0.64–6.41) and <32 weeks’ gestation (10% vs 8%; RR, 1.20, 95% CI, 0.67–6.41).40 Interestingly, a trial involving 137 women with twin pregnancies identified with short cervix ≤25 mm at 18–22 weeks’ gestation reported that spontaneous PTB at <34 weeks’ gestation was significantly reduced in the pessary group than in the expectant management group (5.9% vs 9.1%; RR, 0.41, 95% CI, 0.22–0.76).41
Antibiotics and infection treatment
Although infection plays a critical role in spontaneous PTB, there is no evidence to suggest that antibiotic is effective in preventing this adverse outcome. Bacterial vaginosis occurs when the Lactobacillus species, which is part of the normal vaginal flora, is substituted with anaerobic bacteria such as Gardnerella vaginalis and Mycoplasma hominis. To ascertain whether women diagnosed with bacterial vaginosis and treated with oral metronidazole and vaginal clindamycin before 28 weeks’ gestation reduces the incidence of preterm labour, a recent systematic review comprising nine studies and a meta-analysis of eight RCTs including 10,513 pregnant women reported that there was no reduction in the incidence of preterm labour with the use of oral metronidazole (OR, 0.94, 95% CI, 0.71–1.25) or vaginal clindamycin (OR, 1.01, 95% CI, 0.75–1.36).42
Tocolytic therapy
Spontaneous preterm labour necessitates the use of tocolytics, they act by reducing uterine contractility and aims at delaying delivery at least, until the administration of antenatal corticosteroids for foetal lung maturation and to recommend for a referral to a tertiary facility with appropriate neonatal intensive care unit.43 Different classes of drugs have been used for tocolysis. Since a standard first-line drug has not been identified, most drugs are currently in use including beta mimetics (eg, ritodrine, terbutaline), magnesium sulphate, prostaglandin inhibitors (mostly indomethacin), calcium channel blockers (eg, nifedipine), nitrates (eg, nitroglycerine), and oxytocin receptor blockers (mainly atosiban). Each tocolytic agent has its own mechanism of action, adverse effects, and administration.44
A large systemic review and network meta-analysis sought to determine the most effective tocolytic agent for delaying preterm delivery. A total of 95 RCTs were included with a mean participant population of 111.9 (range 20-708). The probability of a 48-hour delay was highest with prostaglandin inhibitors (OR, 5.39, 95% credible interval [CRI], 2.14–12.34) followed by magnesium sulphate (OR, 2.76, 95% CRI, 1.58–4.94), calcium channel blockers (OR, 2.71, 95% CRI, 1.17–5.91), beta mimetics (OR, 2.41, 95% CRI, 1.27–4.55), and oxytocin receptor blockers (OR, 2.02, 95% CRI, 1.10–3.80) compared with placebo.44
PROFESSIONAL GUIDELINES
Clinical practice guidelines offer a pragmatic approach to guide clinicians in the prevention of preterm labour and delivery. We refer to three key professional guidelines for consideration. The American College of Obstetricians and Gynaecologists (ACOG) recommends that women with singleton pregnancy and prior spontaneous PTB should be offered progesterone supplementation at 16–24 weeks’ gestation regardless of transvaginal cervical length. Furthermore, vaginal progesterone is recommended as a treatment option in asymptomatic women with a single pregnancy without prior PTB and an incidental finding of short cervix of <20 mm before or at 24 weeks’ gestation. Although universal cervical length screening is not mandated for women without history of spontaneous PTB, it may however be considered. Vaginal progesterone is not recommended for multiple pregnancies.45 The ACOG further recommends that women with singleton pregnancy with prior spontaneous PTB and short cervix of <25 mm before 24 weeks’ gestation who do not meet the criteria for cervical insufficiency, may benefit from cervical cerclage placement.7
In the UK, the National Institute for Health and Care Excellence recommends a choice of either prophylactic vaginal progesterone or cervical cerclage for women with prior spontaneous PTB or mid-trimester pregnancy loss between 16 and 34 weeks’ gestation and those with short cervix of <25 mm identified between 16 and 24 weeks’ gestation. For women with short cervical length of <25 mm, in the absence of history of spontaneous PTB or mid-trimester pregnancy loss, clinicians may offer prophylactic vaginal progesterone but not cervical cerclage. Furthermore, for women with short cervix of <25 mm between 16 and 24 weeks’ gestation with either a history of PPROM or cervical surgery, prophylactic cervical cerclage is recommended.46
The Society of Obstetricians and Gynaecologists of Canada (SOGC) recommends that asymptomatic women with a history of PTB who are diagnosed with short cervix of <25 mm at <24 weeks’ gestation should be offered cervical cerclage.46 With regard to progesterone, SOGC recommends that women with a history of PTB be offered intramuscular 17-OHPC 250 mg weekly or vaginal progesterone 100 mg daily. While women with a short cervix of <15 mm at 22–26 weeks’ gestation should be offered vaginal progesterone 200 mg daily.47
CONCLUSION
Prevention of PTB is currently one of the major goals in obstetrics. Though extensive research has been undertaken to understand the underlying pathophysiology of the syndrome with the aim to identify preventative measures, the rate of PTB is not declining. Currently, the use of cervical cerclage and progesterone for the prevention of spontaneous PTB is recommended by professional bodies. However, these preventative measures are not without shortcomings and the target population for prevention is mainly women with a history of PTB with or without short cervix, which constitutes a small number of women that could benefit from interventions. Therefore, the impact of the proposed prevention strategy on the overall rate of spontaneous PTB is limited. For optimal prevention of spontaneous PTB, risk stratification should aim at combining risk factors with different screening tools, such as transvaginal ultrasonographic assessment of cervical length and biomarkers, to identify women at risk of this complication in order to instigate timely prophylactic measures. More research is required to discover potential biomarkers to improve the predictive power of existing risk stratification strategies. The development of an effective method for screening will stimulate further research for the discovery of targeted preventative measures.
About the authors
Dr Kubi Appiah is a PhD student in the Department of Obstetrics and Gynaecology at the Chinese University of Hong Kong, Hong Kong. Conflict of interest: none.
Dr Piya Chaemsaithong is a clinical lecturer in the Department of Obstetrics and Gynaecology at the Chinese University of Hong Kong, Hong Kong. Conflict of interest: none.
Prof Liona Chiu Yee Poon is a clinical professor in the Department of Obstetrics and Gynaecology at the Chinese University of Hong Kong, Hong Kong. Conflict of interest: none.